Explore our solutions
Nucleic acid isolation
Reliable nucleic acid isolation with chemagic technology
Our chemagic technology offers a proven solution for high-quality nucleic acid extraction, developed over 25 years of innovation. Utilizing proprietary M-PVA magnetic beads and specialized separation technology, our system ensures high yields, purity, and minimal cross-contamination. With flexible sample volumes and support from our dedicated application and automation teams, chemagic provides consistent results for diverse downstream applications in gene and cell therapy development.

M-PVA Magnetic Beads
chemagic Applications

chemagic Kits

chemagic Instruments
AlphaLISA AAV capsid detection kits
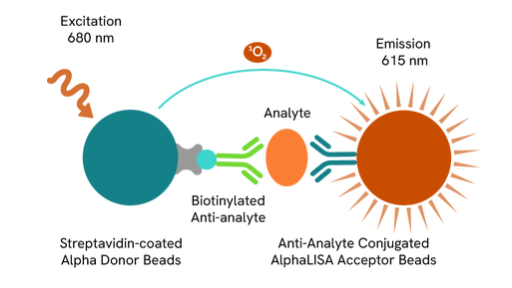
Quantify AAV capsids with AlphaLISA no-wash immunoassays
AlphaLISA technology allows the detection of analytes in a no-wash quantitative assay.
AAVs are widely used as viral vectors in human gene therapy implementing recombinant DNA technology. Measurement of AAV concentration in complex biological matrices is essential for and effective manufacturing of AAV gene therapies.
We have developed and manufactured a line of immunoassays that detect and quantify AAV capsid with AlphaLISA technology. These assays can detect AAV1, AAV2, AAV3B, AAV5, AAV6, AAV8, and AAV9 serotypes, and measure AAV particles present in cell culture media, lysis buffer, and cell lysate.
Alpha Assays
LabChip AAV empty/full characterization
Improving your AAV empty/full analysis
Quantifying adeno-associated virus (AAV) titers and determining the ratio of AAV empty particles pose significant challenges in the development and manufacturing processes. Fast, easy, and low sample volume analysis is critical to ensure efficiency. By optimizing your AAV characterization workflow with the LabChip™ GXII Touch™ characterization system, you can achieve more reliable results, reduce workload, and draw meaningful conclusions about AAV product quality.





























