Determining a direct cell count of tumor cells during live cell analysis of various Immuno-oncology assays
- Directly image cells within a well
- Perform non-invasive, non-toxic, non-radioactive ADCC based assays
- Acquire direct cell counts per well and export all data as a .CSV file
Obtain a direct cell count using the Celigo image cytometer
The Celigo image cytometer is a plate-based cytometer developed for live cell analysis of immune cells. These functional assays scan the entire well of standard microplates and capture brightfield and fluorescent images. The captured images are analyzed with the Celigo software to measure size, morphology, direct cell count, confluence, and fluorescent intensity. The measured parameters are used to generate cell proliferation kinetic data, GFP/RFP expression, tumor spheroid size change, DNA cell cycle analysis, apoptosis, ADCC and CDC cytotoxicity results.
Using Calcein AM to obtain a direct cell count

- Uses Celigo to capture and analyze brightfield and green fluorescent images
- Target suspension and adherent cells are stained with Calcein AM and then mixed with the effector cells
- A direct cell count of target cells (Calcein+) is done and monitored over time
- Reduction in target cell number indicates cell-mediated or antibody-dependent cell-mediated cytotoxicity

For each well, Celigo image cytometer produces high-resolution whole well images for 96-, 384- and1536-well plates.
Celigo image cytometry for direct cell counting in immuno-oncology

Imaged tumor cells stained with Calcein AM
Green Calcein AM stained target cells
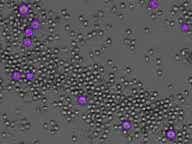
Counted Calcein positive (+) live tumor cells
Outlined counted live target cells
Live cell analysis of immune cells include cytolytic cells: CIK cells, NK cells, Neutrophils and CAR-T cells.
Various fluorescent probes and stains were used to identify the target cells and thereby monitor cell killing. They include fluorescent proteins – GFP, RFP, Calcein AM, Cell tracer dyes CFSE, CellTraceTM dyes and viability dyes – PI, DAPI.
Perform a direct cell count by staining target cells with Calcein AM and using the Celigo image cytometer
| Detection method | Description | Existing issues |
|---|---|---|
| Radioactivity release | Measure the release or radiolabels, 51Cr, 101In in the supernatant | Handling hazardous material; Indirect measurement of cell death |
| Fluorescence release | Measure the release of Calcein AM fluorescent molecules in the supernatant | Indirect measurement of cell death; Endpoint assay only |
| LDH release | Measure the release of cytosolic enzyme in the supernatant | Indirect measurement of cell death; Endpoint assay only |
| Luciferase reporter assay | Measure luciferase as the cells die | Indirect measurement of cell death |
| Flow cytometry | Measure the number of viable cells and viability in the sample | Cannot perform in plates; Must trypsinize for adherent cells |
Cytotoxicity assays play a central role in studying the function of immune effector cells such as cytolytic T lymphocytes (CTL) and natural killer (NK) cells. Traditionally, cytotoxicity assays have been performed using Chromium-51 (51Cr) and Calcein release assays.
The assays involve labeling tumor cells (target) with a radioisotope or fluorescent dyes. When the target cells are subjected to CTLs or NK cells (effector) mediated killing, they release the entrapped labels into the media. The amount of released label in the media is measured to determine the level of cytotoxicity the effectors have induced.
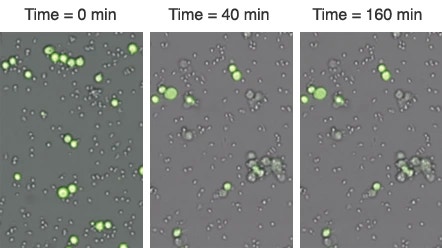
A live cell analysis of cytotoxicity assay using the Celigo image cytometer for direct cell counting of fluorescently labeled target cells has recently been introduced.
Cell images are used by the Celigo software to obtain a direct cell count of tumor cells for each well over multiple time points.
The same plate was imaged over a 160 minute period to monitor the killing of Calcein AM stained tumor target cells by the immune effector cells.

Direct cell counts for each well are reported in the plate map format and counts per well are displayed as a plate map format for easy review.
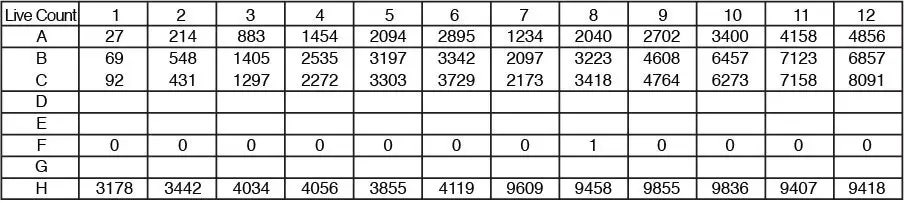
Numerical data per well can be exported to excel as a CSV file. Export all data to Excel. Exported data maintains plate map format within excel for easy data processing
For research use only. Not for use in diagnostic procedures.




























