
Introduction to adipocyte analysis
Adipocytes, also known as fat cells, are important in many biological studies. The size and number of adipocytes are directly associated with adipose tissue metabolism. Due to their fragile nature and large size, adipocytes have historically been difficult samples to work with. Because adipocytes store energy, they are used in many metabolism, diabetes, and obesity studies. Additionally, because lipoaspirates are used as a source of adipose-derived mesenchymal stem cells, separation of immune cells from adipocytes may also be important.
Measuring cell concentration and cell size is often performed manually or by using external software post-microscopy. With a traditional manual hemocytometer method, there is a technical challenge in distinguishing intact adipocytes from lipid droplets generated from ruptured adipocytes in the collagenase digestion process.
Cellometer cell counters incorporate both total cell counting and fluorescent detection in one instrument. In less than a minute per sample, the system can generate reliable cell counting results and also report cell sizes. Various primary adipocytes, such as human, mice, rat, etc. with diameters of 25µm to 250µm can be easily loaded into a disposable counting chamber designed especially for adipocytes to reduce liquid handling reliability issues.
Cellometer instruments for performing adipocyte measurement
Cellometer Spectrum, Auto 2000 and K2 can perform adipocyte measurement: using specialized (PD-300) counting slides to accommodate large cells. The controlled counting chamber depth reduces potential problems caused by the buoyancy of adipocytes. Various primary adipocytes, such as human, mice, rat, etc. with diameters of 25 µm to 250 µm can be loaded into a disposable counting chamber designed especially for adipocytes to reduce liquid handling reliability issues. 55 µl are needed to load the counting chamber slide. Imaging directly from the counting chamber reduces the shear-stress present in flow-based systems where cells travel through the instrument, making accurate analysis of fragile adipocytes possible.

Simple user-friendly procedure
Adipocyte brightfield detection and cell size measurement
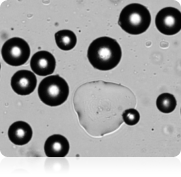
Brightfield image
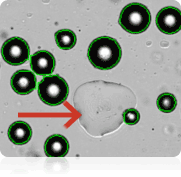
Counted brightfield image
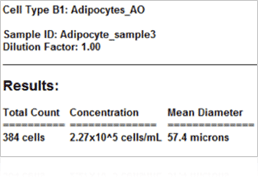
Adipocyte results
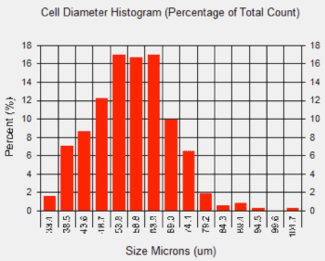
Cell size histogram
In many studies, measuring adipocyte cell size is an important aspect of setting-up baselines or obtaining measurements from downstream experiments, such as looking at the relationship between cell size and energy and glucose metabolism. The images shown above were acquired on the Cellometer Image Cytometer using brightfield detection. The Cellometer software outlines each counted cell in green. In the counted brightfield image, only adipocytes are counted, and the lipid droplet (show with an arrow) is not counted. A cell diameter histogram is generated, showing the cell-size distribution of counted adipocytes. After cell counting is completed, the results show the number of cells counted, the concentration, and the mean cell diameter.
Measuring adipocyte viability using propidium iodide
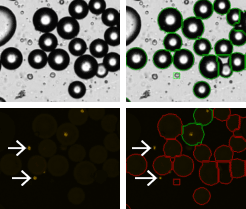
Adipocytes treated with PI
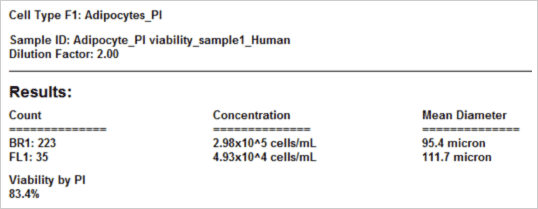
Results reported by Cellometer
The viability of the collected adipocyte sample is crucial for determining the health of the sample. Either for treated or untreated samples, accurate viability measurement could determine how the samples are processed or data is interpreted. Unlike conventional cells, the nucleus in an adipocyte is often located on the periphery of the cell. To obtain the percent viability, all the cells are first counted in bright field (green circles). Because PI is a membrane exclusion dye, only the dead PI-stained cells are counted in the fluorescent image shown with green circles. Live cells, which are PI-negative and are not counted, are shown with the red circles. Furthermore, since free nuclei are not part of whole cells (which we can verify by looking at the brightfield image) they are excluded from the dead cell counts (white arrows). As a result, the Cellometer software reports the number of counted live and dead cells, the concentration, and the mean cell diameter of each population as well as the percent viability.
Detection of adipocyte enzymatic activity using Calcein AM
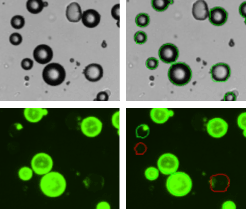
Adipocytes treated with Calcein AM
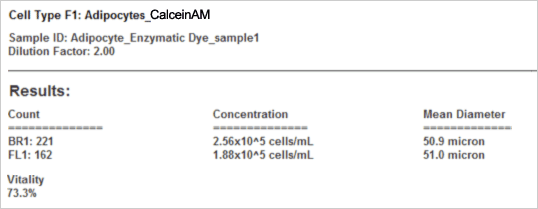
Results reported by Cellometer
The vitality of the cell sample is measured by staining the cells with Calcein AM. Calcein AM (Calcein acetoxymethyl ester) is a cell permeable, non-fluorescent compound. Upon crossing the cell membrane, Calcein AM is rapidly hydrolyzed by cellular esterases inside live cells. The hydrolysis cleaves the AM group, converting the non-fluorescent Calcein AM to a strongly green fluorescing Calcein. Cells that do not possess active cytoplasmic esterases are unable to convert Calcein AM to Calcein, and therefore do not fluoresce green (red-circles in the fluorescent image). This allows for a quick and easy detection of metabolically active cells in a sample. Once the counting is complete, the Cellometer software reports the total number of counted cells in brightfield and the number of cells that are metabolically active, the concentration, and the mean cell diameter of each population as well as the percent vitality of the sample.
Detection of GFP-labeled adipocytes
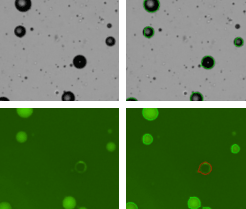
GFP-labeled adipocytes

Results reported by Cellometer
Some laboratories are interested detecting and counting the number of GFP-labeled adipocytes. The strongly green fluorescing cells are detected and counted (green outlined circles) while the weak and non-fluorescing adipocytes are not counted (red outline circles). Once the counting is complete, the Cellometer software reports the total number of counted cells in brightfield and the number of cells that are GFP positive, the concentration, the mean cell diameter of each population, as well as the percent of GFP positive cells.
Publications using Cellometer for adipocyte analysis
- Lee JH, Kirkham JC, Austen WG Jr, et al. (2012) A novel approach to adipocyte analysis. Plastic and reconstructive Surgery 129(2):380-7
- Kvetnansky R, Ukropec J, Vargovic P et al. (2012) Stress stimulates production of catecholamines in rat adipocytes. Cellcular and Molecular Neurobiology 32(5):801-13
- Angle BM, Do RP, Taylor JA, et al. (2013) Metabolic disruption in male mice due to fetal exposure to low butnot high doses of bisphenol A (BPA): Evidence for effects on bodyweight, food intake, adipocytes, leptin, adiponectin, insulin andglucose regulation. Reproductive Toxicology 6238(13)00231-1
Publications using Cellometer for research on adipocyte cell fractions
- AA Ismail, S Wagner, H Murua Escobar, et al. (2012) Effects of high-mobility group a protein application on canine adipose-derived mesenchymal stem cells in vitro. Veterinary Medicine International Vol 752083: 1-10
- Kajiya T, Ho C, Kurtz TW, et al. (2011) Molecular and cellular effects of azilsartan: a new generation angiotensin II receptor blocker. Journal of Hypertension 29(12): 2476-83
- Carvalho PP, Gimble JM, Reis RL, et al. (2010) Human Adipose-derived Stromal/Stem Cells: Use of Animal Free Products & Extended Storage at Room Temperature. Semana de Engenharia
- Jingwei L, Kai T, Xiaoyan L, et al. Cellometer cytometry in adipose-derived stem cells in the application of quantitative analysis. (2012) Chinese Journal of Aesthetic and Plastic Surgery 23(3)
- Renzi S, Lombardo T, Dessì SS, et al. (2012) Mesenchymal stromal cell cryopreservation. Biopreservation and Biobanking 10(3): 276-281
- Carvalho PP, Wu X, Gimble JM et al. (2011) Use of animal protein-free products for passaging adherent human adipose-derived stromal/stem cells. Cytotherapy 13(5):594-7
- Guercio A, Di Marco P, Piccione G, et al. (2012) Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biology International 36(2):189-94
For research use only. Not for use in diagnostic procedures.




























