
Yeast overview
Importance of yeast analysis
Yeasts are an economically important organism used for ethanol production, in the beverage and alternative fuels industries as well as a leavening agent in the baking industry. In addition, pathogenic strains of yeasts are involved in both plant and animal diseases. Concentration and viability determinations are routinely performed for quality control purposes in yeast production, fermentation processes, and fungicides research to monitor proliferation of pathogenic yeasts.
The most common method for determining yeast cell number and viability is manual counting on a standard microscope using a hemacytometer and a viability dye. One advantage of this method is that visual inspection of each sample allows the operator to check for contamination, presence of interfering debris, and obvious dilution errors. A major disadvantage is that the manual method is laborious, error-prone and the data acquired is not easily traceable. Although the equipment used to perform manual counting are relatively inexpensive, the cost of human labor and counting errors can be high enough to render manual counting less than practical in a production facility where accuracy, consistency, and record-keeping are highly desirable.
Fluorescence based image cytometry method for yeast concentration & viability measurement

1. Pipette 20 µl of sample

2. Insert slide
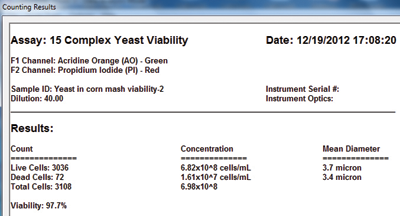
3. Click count and get results
How does yeast count & viability by dual fluorescence work?

A highly viscous corn mash sample is mixed with a dilution buffer and stained with nucleus staining dyes.
Live nucleated cells emit green fluorescence when excited by blue light.
Dead cells emit red light when excited by green light.
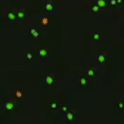
Live and dead cells are then distinguishable by color and viability is generated as a percentage based on live/total cell count.
Yeasts used in brewing industry
In general, yeast used in brewing are very clean and easily counted using Cellometer image cytometer brightfield capability. In addition, in order to measure the viability of the yeast sample, propidium iodide (PI) or oxonol are fluorescent viability dyes that can stain dead cells. Therefore, the total cell count is measured in bright field images and dead cell count is measured in the fluorescent images.
Yeast concentration measurement by brightfield analysis
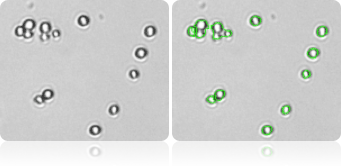
Single cell count
Automatically measure yeast cell size and concentration using Cellometer image cytometer.
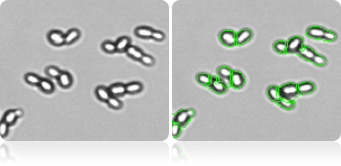
De-clustering of yeast cells
Advanced software de-clustering algorithm can de-cluster yeasts to individual cells to generate more accurate concentration and size measurement.
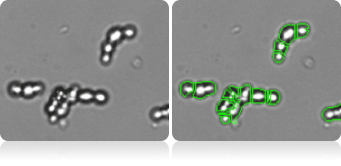
Chain-forming cell count
Advanced software de-clustering algorithm can also de-cluster chain-forming yeasts to individual cells to generate more accurate concentration and size measurement.
Cell concentration linear range and reliability
Serial two-fold dilutions of rehydrated yeast were counted on the Cellometer Auto X4 (10x)* to determine the linear range for reliable yeast concentration measurements. The range of concentration that can be measured on the instrument is dependent on the size of the cell being counted (i.e. the range decreases as cell size increases). The Muntons strain is approximately 4.5 µm in diameter when rehydrated, and was selected for this experiment due to its intermediate size when compared to other yeast strains such as those used for lager beer or wine production. The upper limit for accurate yeast counting, as determined by examining counted images, represented a four-fold dilution of the original concentrated yeast suspension (Dilution 2) at 6.6 x 107 cells/mL. Serial dilutions of this sample were counted until the lower limit was reached (Dilution 6) at a concentration of 4.17 x 106cells/mL.
Plotting of the measured concentration versus the concentration factor (inverse of the dilution factor) resulted in a linear relationship with an R2 of 0.9968. Thus the linear range for counting Muntons yeast (and other similarly sized strains around 4.5 µm in diameter) on the Cellometer Auto X4 (10x)* is between 4 x 106 and 6.6 x 107 cells/mL (Figure 1A).
Repeated measurements were performed on the sample to determine the reliability of counting by calculating the Coefficient of Variation (CV). Within the linear range for counting yeast on the Cellometer Auto X4 (10x)*, the CV was between 4% and 13% (Figure 1B).
*The Cellometer Auto X4 (10x) has been superseded by the Cellometer Spectrum instrument.
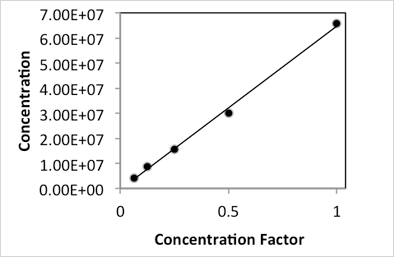
Figure 1A
| Dilution | Mean Concentration | CV |
|---|---|---|
| 2 | 6.58E+07 | 6% |
| 3 | 2.99E+07 | 4% |
| 4 | 1.55E+07 | 7% |
| 5 | 8.84E+06 | 10% |
| 6 | 4.17E+06 | 13% |
Figure 1B
Linear range of accurate yeast concentration measurements and corresponding CV values.
Yeast concentration & viability measurement by brightfield & fluorescence
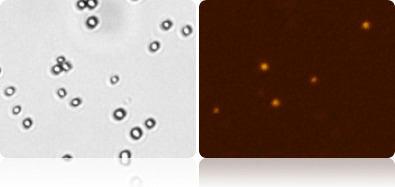
Viability Measurement Using Propidium Iodide (PI)
Munton’s yeasts were allowed to rehydrate for 30 min, and then stained 1-to-1 with propidium iodide (using FOM VB-595-502 or VB-660-503). The yeasts were immediately analyzed using Cellometer to measure the viability, concentration, and cell size. The brightfield images were counted to measure total cell count, while the fluorescent images were counted to measure dead cell count.
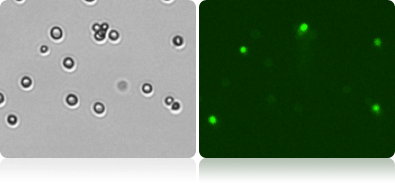
Viability measurement by Oxonol
Munton’s yeasts were allowed to rehydrate for 30 min, and then stained 1-to-1 with oxonol (using FOM VB-535-402). The yeasts were analyzed after 5 min incubation, using Cellometer to measure the viability, concentration, and cell size. The brightfield images were counted to measure total cell count, while the fluorescent images were counted to measure dead cell count.
Cell viability measurement accuracy and reliability
Aliquots of the live yeast suspension were mixed with the heat-killed yeast to generate samples at various levels of viability. The measured viability of the live yeast sample was 78% while the heat-killed sample was 0%. Fractions of live and dead yeast were mixed to analyze intermediate viability levels.
The measured viability was plotted against the percent of heat-killed cells in the final mixture and gives a linear correlation with an R2 of 0.9996. Comparison of predicted and measured viability showed a high degree of agreement and replicates of viability measurements had a standard deviation of ≤ 1.3% (Figure 2A,B). Taken together, viability measurements using PI on the Cellometer Auto X4 (10x) are both highly reproducible and reliable.
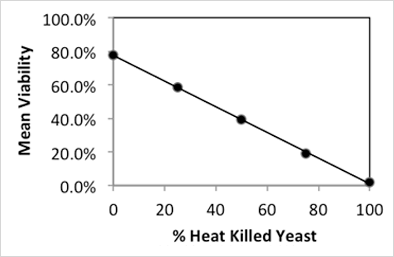
Figure 2A
| Live/Dead | Predicted | Measured | St Dev |
|---|---|---|---|
| 100%/0% | 78% | 0.4% | |
| 75%/25% | 59% | 58% | 1.2% |
| 50%/50% | 39% | 39% | 0.6% |
| 25%/75% | 20% | 19% | 1.3% |
| 0%/100% | 0% | 0% | 0.4% |
Figure 2B
For research use only. Not for use in diagnostic procedures.




























