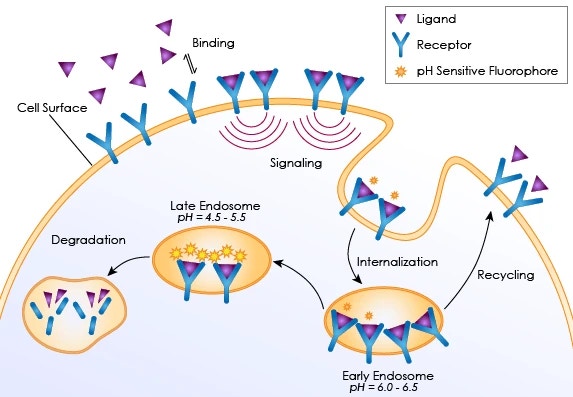
Internalization introduction

Receptor internalization assays measure the absorption of membrane receptors into the cell via endocytosis. The event is activated by the binding of ligand to surface receptors that signal the formation of plasma membrane-formed inward vesicles to enclose the target receptors. After the vesicles are formed and internalized, they are redirected to fuse with early endosomes (pH 6.0-6.5) that can recycle the receptors back to the plasma membrane, or they can be degraded via late endosomes and lysosomes (pH 4.5-5.5).
Traditionally, live cell analysis of receptor internalization can be qualitatively inspected via fluorescent microscopy to observe fluorescently-labelled receptors such as Fc and G-protein coupled receptors (GPCRs). Furthermore, western blotting can also be utilized to quantify the level of receptor internalization. However, these methods are time-consuming and can require experienced researchers to obtain meaningful results.
By using the Celigo™ imaging cytometer, antibody or fluorescent protein-labelled receptors can be imaged and the fluorescent intensities of the target cell populations measured to determine the level of receptor internalization automatically.
Celigo image cytometry
Experimental method
- Target cells (THP-1) are seeded at 2 x 104 cells/well in a 24-well plate (Greiner 662160)
- Cells Fc receptors are labelled with IgG-Dylight-488 or IgG-pHrodo, a pH sensitive dye that fluoresces in an acidic environment (endosome and lysosome)
- Cells in experimental wells are treated with Compound A, which should promote receptor internalization
- Cells in control wells are treated with Compound B, which should not promote receptor internalization
- The cells are incubated for 24 hours
- After incubation, the plate is scanned with the Celigo image cytometer and the images are analyzed to measure % internalization
Receptor internalization fluorescent images
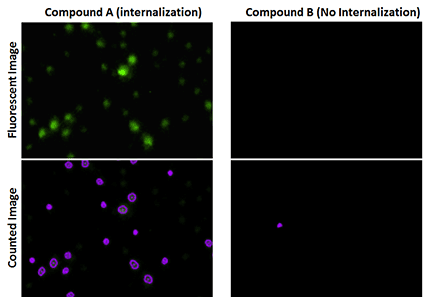
Fluorescent images showed a high percentage of receptor internalization of cells treated with Compound A.
The green fluorescence occurs when vesicles of invaginated receptors are fused with late endosomes and lysosomes.
The total number of cells are counted using the Celigo-captured brightfield images, while the fluorescent images are counted to determine the number of cells showing internalization.
The percentage internalization is then calculated by # fluorescent cells/total.
Quantification of percentage internalization using the Celigo Image cytometer

Cells treated with Compound A showed a high percentage of receptor internalization compared to the control Compound B.
In addition, pHrodo fluorescence showed a higher percentage internalization compared to Dylight-488.
Conclusion
The Celigo image cytometer can be used to measure percentage receptor internalization via fluorescence detection.
The ability to capture brightfield and fluorescent images in a multi-well microplate automatically allows for high-throughput cell-based assays.
In addition, analyzing adherent cells directly on the plate eliminates the need for trypsinization and removes the disruption to the cells.
Phagocytosis introduction
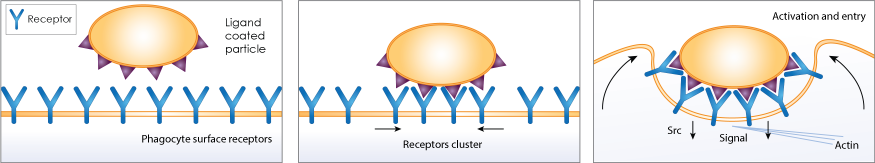
Phagocytosis is an essential process of the immune system to eliminate cellular debris and pathogens. It is a specific form of endocytosis involving vesicular internalization. Phagocytic cells such as macrophages can be attracted to pathogens or cellular debris and engulf the material to be trapped in an internal vesicle called a phagosome. The phagosome fuses with the lysosome to form the phagolysosome, where enzymes and toxic peroxides can digest the pathogen or cellular debris at a low pH value. The host-defense activation of phagocytosis is by attachment of mammalian immune cells to pathogen-associated molecular patterns (PAMPS), which can lead to NF-κB activation.
Traditionally, live-cell analysis of phagocytosis can be qualitatively examined via standard optical microscopy to observe the engulfment of bacteria or cellular debris. In addition, phagocytosis can also be quantitatively measured by incubating phagocytes with particles bound with serum or IgG. After incubation, the excess particles are washed away, leaving only phagocytes with engulfed particles. These cells are lysed and release the engulfed material, which can then be examined by using a microplate reader or by western blot analysis to quantify the level of phagocytosis. However, these methods are time-consuming and can require experienced researchers to obtain meaningful results.
By using the Celigo image cytometer, phagocytosis can be measured using a pH sensitive label called pHrodo. The labels will only fluoresce when they are trapped in the phagolysosome where the pH values are low. Therefore, by measuring the amount of fluorescence or percentage of cells fluorescing, the Celigo system can be used to determine the fluorescent intensities of the target cell populations and measure the level of phagocytosis.
Celigo imaging cytometry
Experimental method
- Target cells (THP-1) are seeded at 2 x 104 cells/well in a 24-well plate (Greiner 662160)
- Cells are stimulated with LPS for differentiation into macrophages
- Cells are incubated for 24 hours
- Two compounds are added to the wells, compound A induces phagocytosis, and compound B is the control
- The cells are then mixed with particles labelled with pHrodo. The pH sensitive dye can fluoresce in acidic environment (endosome and lysosome)
- The cells are incubated for 24 hours
- After incubation, the plate is scanned with the Celigo image cytometer and analyzed to measure percentage internalizatio
Phagocytosis Dextran pHrodo fluorescent images
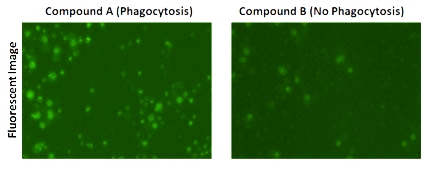
Fluorescent images showed a high percentage of cells with Dextran pHrodo green fluorescence when treated with Compound A.
Fluorescent images also showed a low percentage of cells with Dextran pHrodo green fluorescence when treated with Compound B, which is the control.
The green fluorescence occurs when particles labelled with Dextran pHrodo are engulfed into the phagosome and fused with the lysosome.
The total number of cells are counted using the Celigo-captured brightfield images, while the fluorescent images are counted to determine the number of cells showing internalization.
The percentage phagocytosis is then calculated by # fluorescent cells/total
Quantification of percentage phagocytosis using the Celigo image cytometer
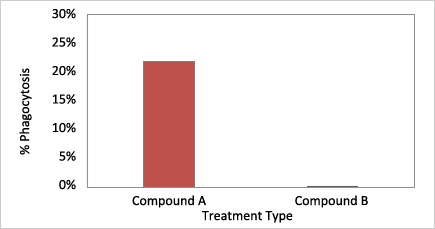
Cells treated with Compound A showed a high percentage of phagocytosis compared to the control Compound B.
Conclusion
The Celigo imaging cytometer can be used to measure percentage phagocytosis via fluorescence detection of Dextran pHrodo dye.
The ability to capture brightfield and fluorescent images in a multi-well microplate automatically allows for high-throughput cell-based assays.
In addition, analyzing adherent cells directly on the plate eliminates the need for trypsinization and removes the disruption to the cells.
For research use only. Not for use in diagnostic procedures.




























