Measuring viral titers by direct cell counting of fluorescently labeled infected cells
- High-throughput automated imaging and analysis directly in plates
- Directly count infected cells in plates without trypsinization
- Image and analyze a 96-well plate in 8 min
Introduction to viral titers
Viral infectivity is highly important to determine the strength of a virus against host cells. In order to determine viral infectivity, common practice is to perform a viral particle titration to infect host cells and measure the infectivity through calculating a dose response. This live cell analysis method can be used on a variety of host cells and virus types to characterize the target virus.
Protocol
- The host cells are seeded and allowed to adhere to the surface of the 96-well
micro plates for 24 hours - Different concentrations or amount of virus particles are added to the plate for
measuring the infectivity of the virus - The infected cells should be labeled with horseradish peroxidase or
fluorescence conjugated antibodies, or fluorescent viral proteins - Live cell analysis of host cell samples is performed using the Celigo™ image
cytometer to count the number of infected cells or percent infection
Measure influenza viral titration with mKate2 fluorescent protein reporter in 96-well plates
- MDCK host cells are seeded in a 96-well micro plate and allowed to adhere overnight
- A 5-fold titration of mKate2-influenza viral particles are prepared and added to the host cells
- The samples are allowed to incubate for 24 hours and then imaged and analyzed
with Celigo image cytometer to count the number of infected cells

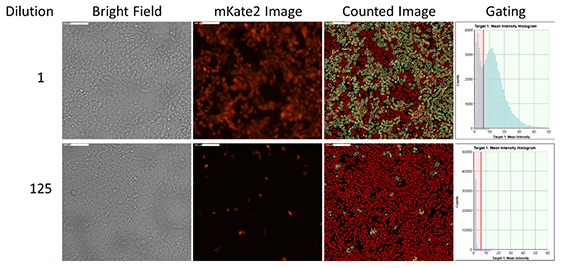
Brightfield and fluorescent images of mKate2-expressing infected cells
Example fluorescent images and gating show the counting results of mKate2-Influenza positive infected cells

Dose-dependent infectivity curve
Utilizing the gating functions in Celigo, the percent of mKate2-Influenza positive cells can be measured to generate an infectivity curve
Measure Zika viral titration with GFP fluorescent protein reporter in 96-well plates
- Protein Reporter in 96-well Plates Vero and Raji host cells are seeded in 96-well micro plates and allowed to adhere overnight
- A 2-fold titration of Reporter Virus Particles (RVP-Green) are prepared and added to the host cells
- The samples are allowed to incubate for 24 hours and then imaged and analyzed
with Celigo image cytometer to count the number of infected cells
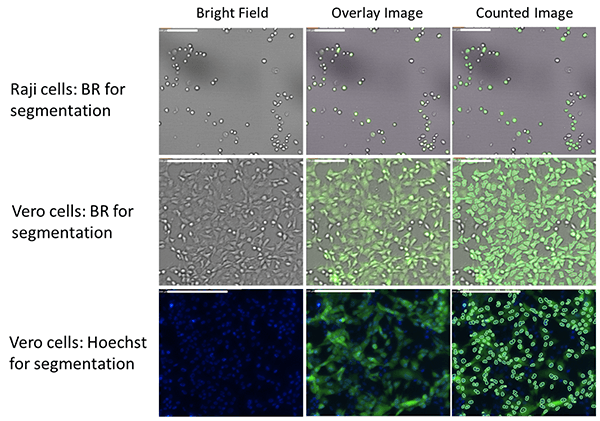
Brightfield and fluorescent images of GFP-expressing infected cells
The Raji cells are first identified in the brightfield and the GFP fluorescence is directly measured in the green channel. The Vero cells are counted in both brightfield or Hoechst fluorescence to determine the GFP fluorescence
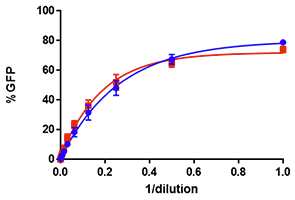
Raji cells
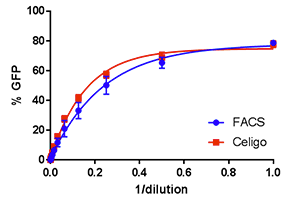
Vero cells
The Celigo percent infectivity results for both Raji and Vero cells are directly compared to FACS showing highly comparable data
For research use only. Not for use in diagnostic procedures.




























