Analysis of cell health using the Celigo™ image cytometer has several advantages:
- Easy-to-use interface to identify cell health
- Rapid scanning and analysis (scan and analyze a 96-well plate using 3 fluorescent channels in <12 min)
- Works in multi-well plate formats (1536-well to 6-well)
- Advanced flow cytometry-like gating
Viability introduction
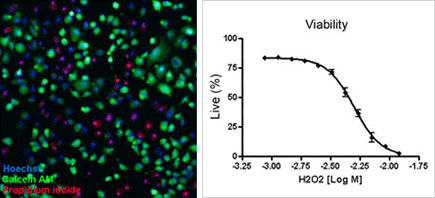
Quick assessment of cell health is performed using the Celigo image cytometer with its easy-to-use applications that allow the simple and rapid analysis of cell viability and apoptosis in many diverse cell types. The Cell Viability application uses Calcein AM, propidium iodide and Hoechst to identify and count live, dead and total cell numbers, respectively. Similarly, the Phosphatidylserine (PS) Externalization application measures apoptosis by quantifying annexin V, propidium iodide and Hoechst to report apoptotic, dead and total cell counts, respectively.
Cell viability application on the Celigo image cytometer

Image of heLa cells & Concentration response curve
Briefly, cells are simultaneously stained with a mixture of Calcein AM, propidium iodide and Hoechst 33342 for staining of live, dead and all cells, respectively. Images are acquired and analyzed using the Celigo software. Markers are identified in each fluorescent channel and for each well of a microtiter plate. Live and dead cell counts as well as the percentage of live and dead cells are automatically reported.
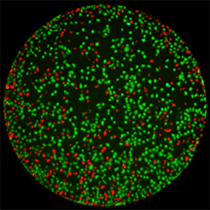
Live / Dead
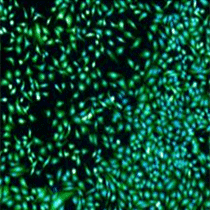
Live / Total
For research use only. Not for use in diagnostic procedures.




























