High-throughput plaque and foci counting in brightfield and fluorescence imaging
- High-throughput automated imaging and analysis directly in plates
- Directly count infected cells in plates without trypsinization
- Image and analyze a 96-well plate in 8 min
Introduction to plaque and foci counting
The ability to quantify viral infectivity by automated counting of plaques and foci is highly important. In order to rapidly and efficiently determine viral infectivity, multiple conditions or titrations need to be tested with the virus and host cells. There are various types of plaques and foci using horse radish peroxidase or fluorescent labeling with markers or proteins. These allow the development of high-throughput counting of foci and plaques.
Protocol
- The host cells are seeded and allowed to adhere to the surface of the 96-well micro plates for 24 hours
- Different concentrations or amount of virus particles are added to the plate for measuring the infectivity of the virus
- Example 1: The infection zone allows clearing of host cells where the host cells are then stained with crystal violet to create contrast between the cells and the opening
- Example 2: infected foci or plaques can be labeled with horseradish peroxidase or fluorescence-conjugated antibodies, or fluorescent viral proteins
- The host cell samples are then imaged and analyzed using a Celigo™ image cytometer to count the number of plaques and foci
Directly counting viral plaques in 6-well microplates
- Vero host cells are seeded in a 96-well microplate and allowed to adhere overnight
- Three different concentrations of viral particles are prepared and added to the host cells
- The samples are allowed to incubate for 48 hours
- After incubation, the host cells are stained with crystal violet, revealing viral plaques (zones of clearing)
- Finally, the plate is imaged and analyzed with Celigo image cytometer to count the number of plaques

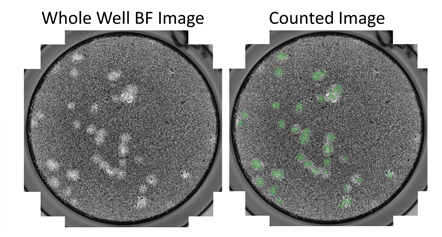
Whole-well brightfield and counted images
Example brightfield images show the counting of viral plaques in 6-well plates
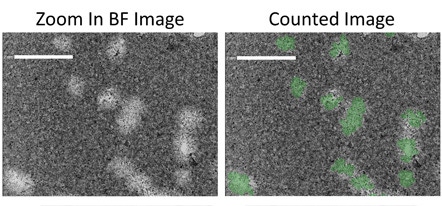
Zoom in brightfield and counted images
Zoomed in example brightfield images show the counting of plaques in 6-well plates
High-throughput quantification of virally induced plaques, counter stained with horseradish peroxidase in 96-well plates
- Host cells are seeded in a 96-well micro plate and allowed to adhere overnight
- Different viral particles are prepared and added to the host cells
- The samples are allowed to incubate for 24 hours
- After incubation, the infected foci are labeled with primary anti-viral protein antibody and a horseradish peroxidase (HRP) conjugated secondary
- Finally, the plate is imaged and analyzed with Celigo image cytometer to count the number of infected plaques

Brightfield and counted images of plaques formed from different viruses
Example brightfield images show the counting of HRP positive infected plaques
High-throughput quantification of fluorescent foci of influenza infected Vero cells in 96-well plates
- Vero host cells are seeded in a 96-well micro plate and allowed to adhere overnight
- A 5-fold titration of influenza viral particles are prepared and added to the host cells
- The samples are allowed to incubate for 24 hours
- After incubation, the infected foci are labeled with primary viral protein antibodies conjugated with Dylight 488
- Finally, the plate is imaged and analyzed with Celigo image cytometer to count the number of infected cells
- Example fluorescent images show the counting of Dylight 488 positive infected cells
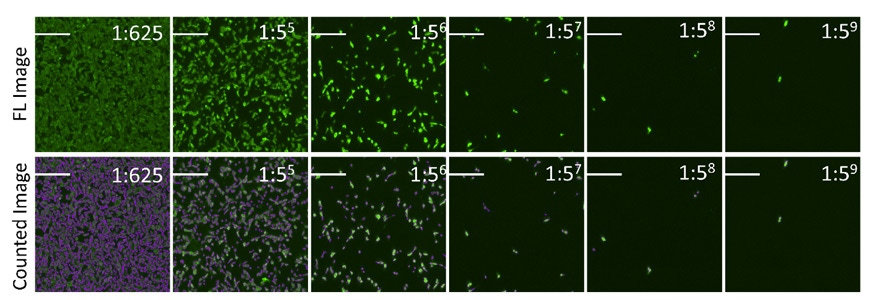
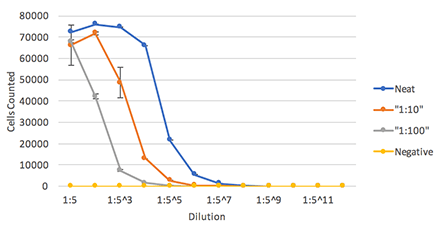
The percent Dylight 488 positive cells are counted to generate an infectivity curve shown below, and the results are compared directly to manual counting, which only covered 10% of the well
Influenza infected foci
| Log10 (FFU/mL) | Celigo | Manual |
|---|---|---|
| Neat | 8.97 | 9.03 |
| 1:10 | 8.05 | 8.03 |
| 1:10 | 7.99 | 7.90 |
| 1:100 | 6.97 | 6.99 |
| 1:100 | 6.99 | 7.04 |
Celigo showed highly comparable plaque counting results to manual counting method.
High-throughput quantification of fluorescent plaques of influenza infected Vero cells in 96-well plates
- Vero host cells are seeded in 96-well micro plates and allowed to adhere overnight
- A 2-fold titration of GFP-influenza virus particles are prepared and added to the host cells
- The samples are allowed to incubate for 24 hours and then imaged and analyzed with Celigo image cytometer to count the number of infected cells
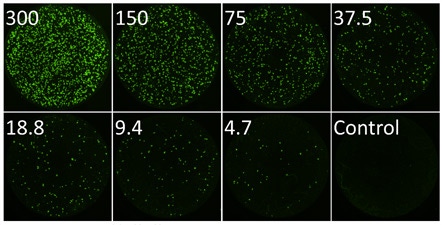
Whole well fluorescent image of infected fluorescent plaques for each dilution
Exampleimages show the whole well imaging of fluorescent viral plaques captured on the Celigo image cytometer. A whole well image was captured for each viral dilution providing a whole well view of the viral infection.
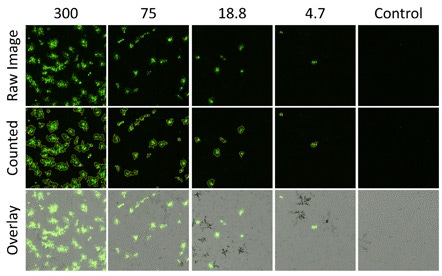
Infected fluorescent plaques directly counted in green fluorescence channel using the Colony Application
Examples of zoomed in images of viral fluorescent plaques captured on the Celigo. The middle row is the viral plaques that have been analyzed by the Celigo software. The identified plaques are automatically circled by the software. The bottom row is showing an overlay of the brightfield and the fluorescent images. The Celigo software allows for the overlay of multiple images from multiple channels to be overlayed with a click of a button.
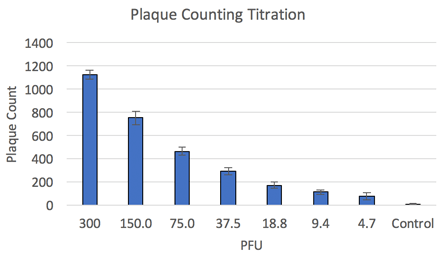
Fluorescent plaque counting results
Once the viral plaques are imaged and counted by the Celigo the data is exported to excel for further analysis. The number of viral plaques is graphed over the performed dilution series of the virus. This data shows that the number of viral plaques is directly related to the amount of virus added to each well.
For research use only. Not for use in diagnostic procedures.




























