Introduction
Measuring the count, concentration, viability and vitality of yeast are the most common measurements performed in yeast production and investigation. By using fluorescence-based detection methods the Cellometer Spectrum instrument can also measure the glycogen content, neutral lipid content, trehalose content and GFP transfection efficiency of cultured yeast samples.
Advanced fluorescent assays measuring yeast physiologic parameters

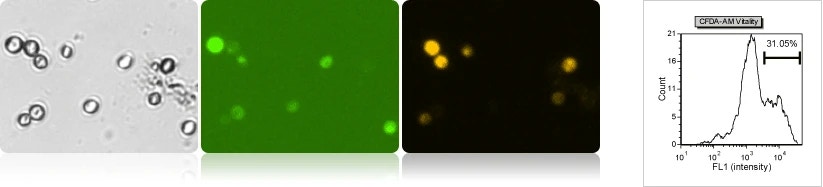
Yeast vitality analysis using CFDA-AM & propidium iodide
The brewing yeasts were stained for 1 hour with CFDA-AM (using FOM S1-534-470) to measure enzymatically active yeasts. After incubation, the cells are immediately analyzed using Cellometer to measure the number of enzymatically active yeasts to determine the vitality of the sample. The percentage of yeasts that are actively fermenting during production can be determined and used to optimize the fermentation process. The fluorescent linear gating was set to measure the population percentage of high CFDA-AM fluorescence.
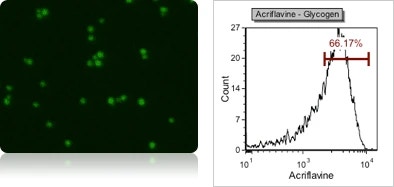
Yeast glycogen content using acriflavine fluorescent staining
Yeast glycogen is a macromolecule that provides the energy and carbohydrates required for yeast sterols and lipid synthesis, which ensures yeast metabolism during fermentation. Glycogen content can be measured by staining with acriflavine (using FOM S1-534-470) and analyze fluorescence intensity of individual cells in a fluorescent histogram. Glycogen has been shown to correlate to vitality and is an essential physiological parameter to be monitored.
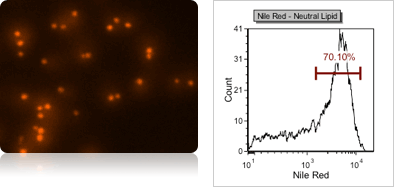
Yeast neutral lipid content using Nile red fluorescent staining
Neutral lipids are energy rich molecules that are stored in yeasts, which cannot be metabolized. However, it can protect yeast cells from toxic substances like ethanol, which can prolong survival capacity. To measure neutral lipids, yeast can be stained with Nile Red (using FOM S1-655-527), and analyze fluorescence intensity of individual cells in a fluorescent histogram. The fluorescent linear gating was set to measure the population percentage of high Nile Red fluorescence.
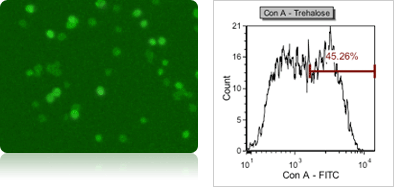
Yeast trehalose content using concanavalin A-FITC fluorescent staining
Trehalose is a sugar disaccharide that supplies energy during cell cycle, or cell proliferation. Like neutral lipids, trehalose can protect yeast cells against stress, high ethanol content, heat, dehydration, oxidation, pH change, and also increase survival capacity during fermentation. To measure trehalose content, the yeast cells are stained with concanavalin A-FITC (using FOM S1-535-470), and analyze fluorescence intensity of individual cells in a fluorescent histogram. The fluorescent linear gating was set to measure the population percentage of high FITC fluorescence.
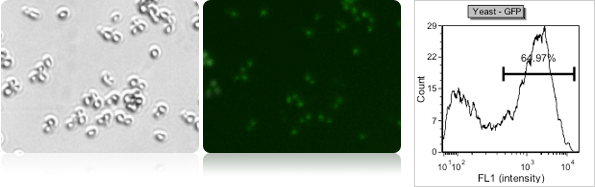
Yeast green fluorescent protein (GFP) transfection
Green fluorescent proteins expression in yeasts can be measured using Cellometer (using FOM S1-535-470). Brightfield images are analyzed initially to count all the cells and the fluorescence intensities within each cell are measured and plotted in a histogram for analysis. The fluorescent linear gating was set to measure the population percentage of high GFP fluorescence.
References
- L. L. Chan, E. J. Lyettefi, A. Pirani, T. Smith, J. Qiu, and B. Lin, “Direct concentration and viability measurement of yeast in corn mash using a novel imaging cytometry method,” J Ind Microbiol Biotechnol, vol. 38, pp. 1109-1115, 2010.
- L. L. Chan, X. Zhong, A. PIrani, and B. Lin, “A novel method for kinetic measurements of rare cell proliferation using Cellometer image-based cytometry,” Journal of Immunological Methods, vol. 377, pp. 8-14, 2012.
- L. L. Chan, X. Zhong, J. Qiu, P. Y. Li, and B. Lin, “Cellometer Vision as an alternative to flow cytometry for cell cycle analysis, mitochondrial potential, and immunophenotyping,” Cytometry Part A, vol. 79A, pp. 507-517, 2011.
- L. L.-Y. Chan, N. Lai, E. Wang, T. Smith, X. Yang, and B. Lin, “A rapid detection method for apoptosis and necrosis measurement using the Cellometer imaging cytometry,” Apoptosis, vol. 16, pp. 1295-1303, 2011.
- Cellometer References for Yeast Analysis
Yeast references
- Liu J, Wisniewski M, Droby S, Tian SP, Hershkovitz V, Tworkoski T. Effect of heat shock treatment on stress tolerance and biocontrol efficacy of Metschnikowia fructicola. Fems Microbiology Ecology;76(1):145-155 2011
- Liu J, Sui Y, Wisniewski M, Droby S, Tian SP, Norelli J, Hershkovitz V. Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biology and Technology;65:61-68 2012
- Xu L, Du Y. Effects of yeast antagonist in combination with UV-C treatment on postharvest diseases of pear fruit. BioControl;57:451-461 2012
- Liu J, Wisniewski M, Droby S, Vero S, Tian SP, Hershkovitz V. Glycine betaine improves oxidative stress tolerance and biocontrol efficacy of the antagonistic yeast Cystofilobasidium infirmominiatum. International Journal of Food Microbiology;146(1):76-83 2011
- Liu J, Wisniewski M, Droby S, Norelli J, Hershkovitz V, Tian SP, Farrell R. Increase in antioxidant gene transcripts, stress tolerance and biocontrol efficacy of Candida oleophila following sublethal oxidative stress exposure. Fems Microbiology Ecology;80(3):578-590 2012
- Sui Y, Liu J, Wisniewski M, Droby S, Norelli J, Hershkovitz V. Pretreatment of the yeast antagonist, Candida oleophila, with glycine betaine increases oxidative stress tolerance in the microenvironment of apple wounds. International Journal of Food Microbiology;157(1):45-51 2012
- Chan LL, Lyettefi EJ, Pirani A, Smith T, Qiu J, Lin B. Direct concentration and viability measurement of yeast in corn mash using a novel imaging cytometry method. Journal of Industrial Microbiology & Biotechnology;38(8):1109-1115 2011
- Berkes CA, Chan LLY, Wilkinson A, Paradis B. Rapid quantification of pathogenic fungi by Cellometer image-based cytometry. Journal of Microbiological Methods 2012 Dec:468-476.
- Chan LL, Kury A, Wilkinson A, Berkes C, Pirani A. Novel image cytometric method for detection of physiological and metabolic changes in Saccharomyces cerevisiae. Journal of Industrial Microbiology & Biotechnology;39(11):1615-1623 2012
Mold references
- Zhang CF, Wang JM, Zhang JG, Hou CJ, Wang GL. Effects of beta-aminobutyric acid on control of postharvest blue mould of apple fruit and its possible mechanisms of action. Postharvest Biology and Technology;61(2-3):145-151 2011
- Wang J, Shi X-G, Wang H-Y, Xia X-M, Wang K-Y. Effects of Esterified Lactoferrin and Lactoferrin on Control of Postharvest Blue Mold of Apple Fruit and Their Possible Mechanisms of Action. J. Agric. Food Chem.;60:6432-6438 2012
- Wang J, Xia X-M, Wang H-Y, Li P-P, Wang K-Y. Inhibitory effect of lactoferrin against gray mould on tomato plants caused by Botrytis cinerea and possible mechanisms of action. International Journal of Food Microbiology;61(3):151-157 2013
Spore references
- Liu J, Macarisina D, Wisniewskia M, Suia Y, Drobyb S, Norellia J, Hershkovitzb V. Production of hydrogen peroxide and expression of ROS-generating genes in peach flower petals in response to host and non-host fungal pathogens. Plant Pathology;10.1111/j.1365-3059.2012.02683.x 2012
- Mishra S, Malik A. Nutritional optimization of a native Beauveria bassiana isolate (HQ917687) pathogenic to housefly, Musca domestica L. Journal of Parasitic Diseases 10.1007/s12639-012-0165-5 2012
Parasite references
- Espinosa A, Paz-Y-Mino-C G. Discrimination, Crypticity, and Incipient Taxa in Entamoeba. Journal of Eukaryotic Microbiology;59(2):105-110 2012
For research use only. Not for use in diagnostic procedures.




























