
Introduction to adoptive cell transfer therapy
Adoptive cell transfer (ACT) therapy focuses of using the patient’s own cells for therapeutic treatment. Within ACT there are three major therapeutic approaches that use cells to directly target and stimulate the immune system: Tumor-infiltrating lymphocyte (TIL), T-cell receptor (TCR), and chimeric-antigen receptor (CAR) therapies. Although the approach to immune system stimulation is different from one therapy to the next, they all use and require primary, purified, and clinical samples. Whether plating cells for an ELISA or proliferation assays or working toward isolation of TCR from humanized mice, these samples are routinely processed and re-processed for downstream experiments. At each step, measurement of cell viability and concentration remains an important element in the immunotherapy research and development process.
Measuring cell concentration and viability
Using Trypan Blue to measure cell concentration and viability
Cellometer instruments are capable of performing the Trypan blue exclusion method. After staining the cells with Trypan blue, and loading the sample into the counting chamber slide, the Cellometer instrument automatically captures and analyzes multiple cell images. Within seconds, the result table displays the live, dead, and total cell concentrations, percent viability as well as the average cell size.
Trypan Blue protocol
- Dilute the stock (0.4 %) with PBS to 0.2 %.
- Filter the Trypan blue with 0.2 micron filter
- Mix the cell suspension at 1:1 with 0.2 % Trypan blue
- Load the counting chamber slide into the Cellometer and analyze
Simple, user-friendly Cellometer procedure

1. Pipette 20µl 2. Insert slide 3. Select assay & click count 4. Results in 30 seconds!
Using Acridine Orange/Propidium Iodide (AO/PI) to measure cell concentration and viability
Dual fluorescence-based instruments (Cellometer Ascend, Spectrum, Auto 2000, and K2) are capable of measuring the concentration and viability of nucleated cells in clinical research samples without the need to perform red-blood cell lysis. 20 µl of sample is added to the Cellometer counting chamber. Imaging and analysis of the samples is completed in 30 seconds or less. Brightfield and fluorescent cell images can be viewed to check cell morphology and verify cell counting. Total cell count, concentration, and mean diameter are automatically displayed for each counted sample.
AO/PI protocol
- Obtain ViaStain™ AO/PI solution
- Stain cell sample at 1:1 with AO/PI solution
- Load counting chamber slide and analyze

1. Pipette 20 µl of sample into a disposable slide

2. Insert slide into the instrument
3. Select assay from a dropdown menu
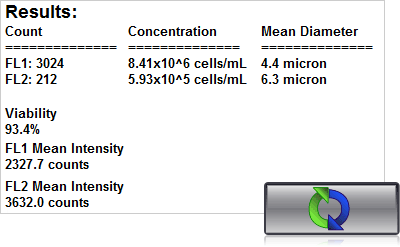
4. Click count, acquire image and view cell count, concentration, diameter
Human and animal cells counted by Cellometer that are used in adoptive cell transfer therapy research and development
Human melanoma digest
The first step in the isolation of tumor-infiltrating lymphocytes (TILs) is the excision and digestion of the tumor sample.
- Use Cellometer to determine concentration and viability of TILs to plate for T-cell isolation post tumor digestion
- Use AOPI to measure cell viability
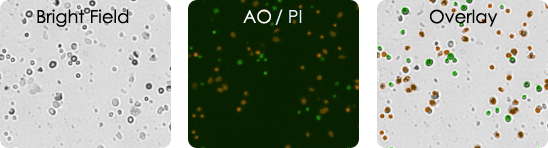
Human melanoma digest sample stained with AOPI
Isolated T-cells
Secretion of IFNγ by TIL indicates tumor specific response by the isolated T-cells. An ELISA assay is performed to test for high levels of interferon-γ (IFNγ).
- Use Cellometer to determine concentration and viability of T-cells needed for an ELISA assay.
- Use AOPI or TB to measure cell viability
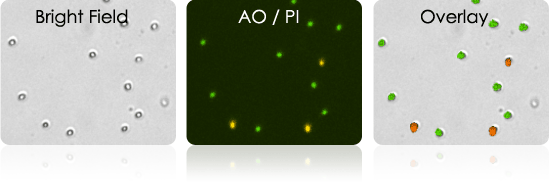
Isolated T-cells stained with AOPI
Harvested Splenocytes
Immunize mice with tumor-specific antigen. Harvest mouse serum and splenocytes for B cell isolation
- Use Cellometer to determine concentration and viability of harvested splenocytes
- Use AOPI to measure cell viability
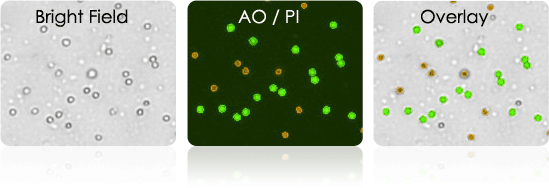
Harvested splenocytes stained with AOPI
Isolated B-cells
Expand selected B-cell clone for hybridoma production.
- Use Cellometer to determine concentration and viability of antibody producing hybridoma B-cells.
- Use AOPI or TB to measure cell viability
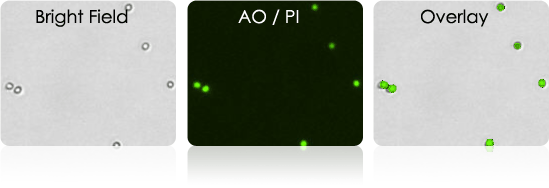
Isolated B-cells stained with AOPI
HEK 293 cells
Using packaging cell line (HEK 293) for the production of the viral vector
- Use the Cellometer to determine the concentration of cells needed to seed for viral vector production.
- Use the Cellometer to determine the concentration of cells needed to seed for testing of the produced viral vector.
- Use AOPI or TB to measure cell viability

HEK 293 cells stained with Trypan Blue
Human PBMCs
Collect human peripheral blood lymphocytes for T-cell isolation.
- Use the Cellometer to determine the viability and concentration of the collected PBMC sample.
- Test sample concentration and viability post Leukapheresis + Ficoll gradient
- Use AOPI to measure cell viability
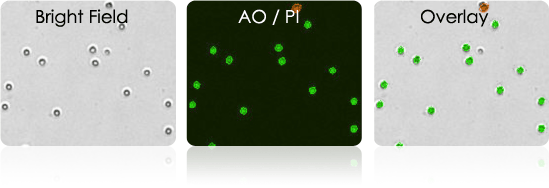
Isolated human T-cells stained with AOPI
Isolated Human T-cells
Purified human T-cells are often cryopreserved before they are transduced or transfected with a vector containing the cancer-specific t-cell receptor.
- Use Cellometer to check the concentration and viability of cells before freeze-down
- Use Cellometer to check the concentration and viability of samples after thawing
- In preparation for transduction/transfection use the Cellometer to check the viability and determine optimal cell concentration for plating
- Use the Cellometer to examine cell viability and concentration during cell expansion
- Use AOPI or TB to measure cell viability
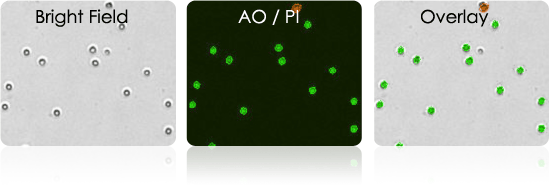
Isolated human T-cells stained with AOPI
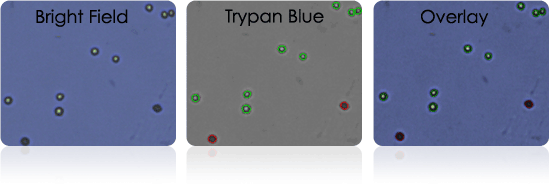
Isolated human T-cells stained with Trypan Blue
Viability detection methods
The Cellometer line of instruments can quickly and reliably obtain the viability of the cell sample by Trypan blue or fluorescent methods. Additionally, the system automatically reports a cell count, concentration, and cell size in a single 20 µl assay. The fluorescent based Cellometer instruments (Ascend, Auto 2000, Spectrum, and K2) also allow researcher to count the total number of nucleated cells in a fresh clinical research sample without the need to lyse red blood cells. The table below outlines the assay that can be used for each selected application.
| Cell type | Viability detection method | |
|---|---|---|
| Trypan Blue | AOPI | |
| Hybridoma cells | ✓ | ✓ |
| 293T cells | ✓ | ✓ |
| T-cells for expansion | ✓ | ✓ |
| Tumor digest | ✓ | |
| Isolated B-cells | ✓ | ✓ |
| Splenocytes – fresh | ✓ | |
| Splenocytes post RBC lysis | ✓ | ✓ |
| PBMC – isolated | ✓ | ✓ |
| PBMC – fresh | ✓ | |
| Feeder cells (K562 cells) | ✓ | ✓ |
Percent of GFP positive cells following transduction/transfection of HEK 293 cells
- HEK 293 cells were cultured in flasks
- Lentiviral vector containing an eGFP reporter was added to the cells at different concentrations: Control (no viral vector), Sample 1, and Sample 2 (images shown below)
- After an incubation period, the cells were collected and processed using a Cellometer
- Percent transduction = (Total GFP+ cells / Total brightfield cells) x 100
| Lentivirus transduction | Total cells | GFP positive | % GFP positive |
|---|---|---|---|
| Sample 1 | 1244 | 718 | 58.0% |
| Sample 2 | 1404 | 337 | 24.1% |
| Control | 1188 | 0 | 0.00% |

Control
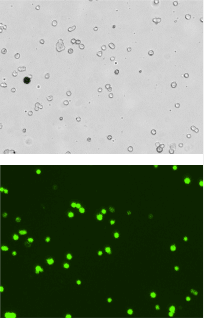
Sample 1
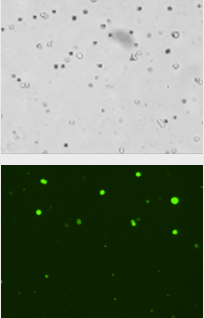
Sample 2
Conclusions
The Cellometer instruments are capable of performing concentration, viability and cell size measurements of cultured and primary cell lines as well as perform more complicated cell-based assays that are used during the research and development of cancer immunotherapies. With brightfield and fluorescent platforms, these robust instruments are capable of performing consistent and reliable cell measurements for a large variety of samples. Dual fluorescence-based units (Ascend, Auto 2000, K2, and Spectrum) are capable of measuring the concentration and viability of nucleated cells in clinical samples without the need to perform red-blood cell lysis. For isolated or purified cell samples the brightfield Trypan blue Cellometer T4 acquires multiple images, and automatically report the percent viability, cell concentration, and average mean diameter in under 30 seconds.
Publications using Cellometer for adoptive cell transfer therapy research
- Talebian L, Wu JY, Fischer DA, et al. (2012) Novel mobilization strategies to enhance autologous immune effector cells in multiple myeloma Front Biosci (Elite Ed) 3: 1500–1508
- Singh H, Figliola MJ, Dawson MJ, et al. (2011) Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Research 71(10): 3516-27
- Manuri PV, Wilson MH, Maiti SN, et al. (2010) piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Human Gene Therapy 21(4):427-37.
For research use only. Not for use in diagnostic procedures.




























