Introduction
The hemocytometer has been an essential tool for hematologists, medical practitioners, and biologists for over a century. The device was initially used by medical practitioners to analyze patient blood samples, which was the initial spark that created the field of hematology. Today the type of cells counted on a hemocytometer have expanded to algae, yeast, cancer cells, stem cells, cultured cells, blood-based cells, parasites, spores and more. Although a variety of automated cell counting instruments have been developed, many researchers still use manual counting with a hemocytometer.
Manual cell counting with Hemocytometer
Step 1. Prepare the Hemocytometer

Clean the hemocytometer and glass cover slip with 70% EtOH.
Step 2. Prep sample & load

Place the glass cover slip over the counting chambers.
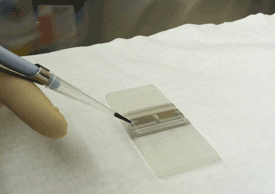
Pipette 10 microliters of cell sample into the hemocytometer.
Step 3. Manually count cells in sample
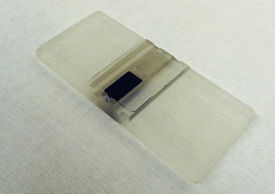
Place the hemocytometer under a microscope with a typical magnification of 100.
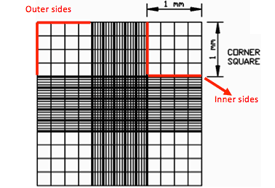
Focus both onto the grid pattern and the cell particles and count the total number of cells found in 4 large corner squares.
If cells are touching the 4 perimeter sides of a corner square, only count cells on 2 sides, either the 2 outer sides or 2 inner sides.
Step 4. Cell calculations & disposal of Hemocytometer
Multiply the dilution factor by the total number of cells, divide by the # of corner squares counted, and multiply by 104 to obtain cell concentration (cells/ml).
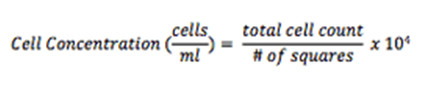
Clean hemocytometer and glass cover slip with 70% EtOH.
Manual cell viability measurement with Hemocytometer
Using Trypan blue to measure mammalian cell viability
One of the earliest and most common methods for measuring cell viability is the Trypan blue (TB) exclusion assay. Trypan blue is a ~960 Daltons molecule that is cell membrane impermeable and therefore only enters cells with compromised membranes. Upon entry into the cell, Trypan blue binds to intracellular proteins thereby rendering the cells a bluish color. The Trypan blue exclusion assay allows for a direct identification and enumeration of live (unstained) and dead (blue) cells in a given population.
Trypan blue viability protocol
Trypan blue preparation
Step 1. Dilute the stock (0.4%) Trypan blue with PBS to 0.2%
Step 2. Filter the Trypan blue with 0.2 micron filter
Hemocytometer preparation
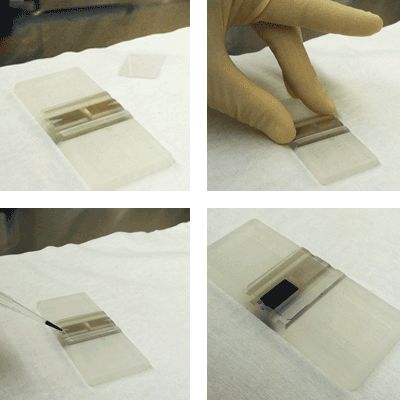
Step 3. Clean the hemocytometer and glass cover slip with 70% EtOH
Step 4. Place the glass cover slip over the counting chambers
Cell counting procedure
Step 5. Vortex the target cell suspension and mix 1:1 with 0.2% Trypan blue
Step 6. Pipette 10 microliters of cell sample into the hemocytometer
Step 7. Wait 30 seconds for the cells to settle
Manually count cells in sample
Step 8. Place the hemocytometer under a microscope using a 10x or a 20x objective
Step 9. Focus both onto the grid pattern and the cell particles, and count the total number of cells found in 4 large corner squares. If cells are touching the 4 perimeter sides of a corner square, only count cells on 2 sides, either the 2 outer sides or 2 inner sides
Step 10. Count the live cells (without Trypan blue) and dead cells (with Trypan blue)
Cell Calculations & Cleaning of Hemocytometer
Step 11. Obtain cell concentration (cells/ml) by multiplying the dilution factor by the total number of cells, divide by the # of corner squares counted, and multiply by 104.

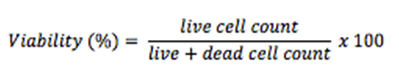
Step 12. Clean the hemocytometer and glass cover slip with 70% EtOH
Similar counting procedures can also be performed using the Revvity disposable hemocytometer.
For research use only. Not for use in diagnostic procedures.




























