Revvity high-throughput platforms can rapidly quantify cell concentration and viability, as well as cell-based assays to streamline the bioprocessing pipeline from engineering host cells to biological protein production.

The three main types of therapy are:
- Biologic protein-based therapy with monoclonal antibodies (mAbs)
- Gene therapy (e.g., Luxturna)
- Cell therapy (e.g., ex vivo CAR-T cell-based therapies such as Kymriah and Yescarta)
Biologic production relies on accurate measurements of total cell concentration and viability at every step to ensure cell survival and effective mAb production. Additional cell fitness characterization can be performed using specific fluorescent reagents such as reactive oxygen species (ROS) or caspase 3/7 apoptosis detection.
Revvity products use advanced image cytometry methods to measure total and viable cell populations, to support consistency and reproducibility in the bioprocessing workflow.
Optimal biologic production depends on:
- Engineering host cells
- Effective cell transfection
- Optimizing cell culture conditions (media, pH, confluence)
- Minimizing variation
- Developing assays to monitor growth and scale-up biologic production
- Monoclonality verification
Historical perspective
The first mAb was created in 1975 after the fusion of a mouse B lymphocyte with a murine myeloma cell; these hybridomas were able to continuously secrete large quantities of antibodies [Joubert et al. 2019]. The 1970s also saw the production of the first genetically engineered human insulin by Escherichia coli.
In 1987, Chinese Hamster Ovary (CHO) cells were introduced to produce mAb, which was a major advancement in mAb manufacturing. This system was used to produce tissue plasminogen activator in 1987, followed by erythropoietin two years later. In the 1990s, the first chimeric recombinant and humanized antibodies were established [Joubert et al. 2019].
Currently, more than half of the available commercial mAbs are made in CHO cells. They offer the advantages of being robust, genetically manipulable, and possessing a strong track record with regulatory authorities.
They can be modified to:
- Improve cell growth
- Minimize apoptosis
- Optimize metabolism
- Promote protein product secretion
Mammalian cells provide the additional benefit of similarity with human glycosylation and other post-translational modifications (PTMs) [Dalton and Barton, 2014]. Approximately 70% of biopharmaceuticals are based on mammalian cell culture processes, which can cause bottlenecks in their production due to limits on cultivation time, growth capacity, and product yield [Fischer et al., 2015].
Despite these challenges, mAbs have become one of the most widely used therapeutic classes. In 2014, they accounted for half of the ten best-selling drugs and $50 billion in sales annually [Baeshen et al. 2014]. While antibody-based drugs are an important tool in the armamentarium against hundreds of medical conditions, several critical biomanufacturing issues are obstacles in the industry, including protein expression platform productivity [Joubert et al. 2019].
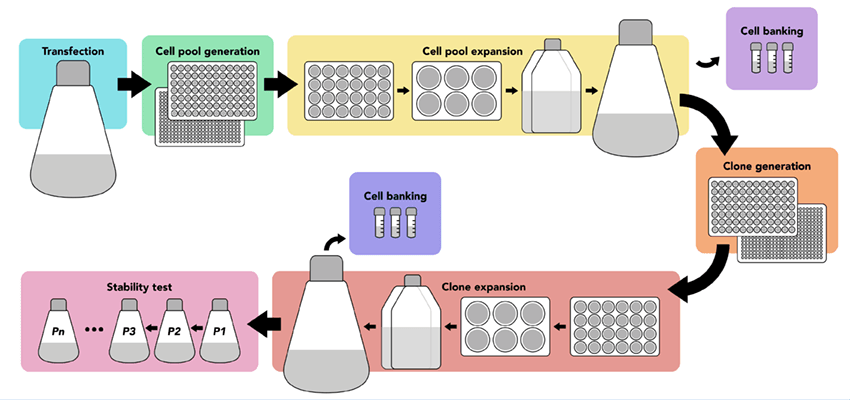
Each of these critical steps relies on accurate measurements of cell concentration and viability. Counting viable cells is necessary for downstream assays of productivity (antibody titer, enzyme activity levels, etc.).
- Engineering CHO cells with CRISPR
- Transfecting cell lines
- Selecting media
- Monitoring growth and viability
- Single-cell cloning
- Clone selection
Current technologies for optimizing bioprocessing workflow
During early-stage research, it is important to define and optimize a reproducible manufacturing process with an established characterization profile to support product release and development.
Efficient production relies on three key checkpoints:
- Total and viable cell concentrations
- Cell viability
- Protein productivity and quality
The first two indicators are critical factors to optimize cell seeding, selecting the best feeding strategy, and timing harvesting. All three variables contribute to both protein productivity and quality in each step of the biologics development process.
Image cytometry assays improve speed and efficiency for cell counting assays
Current challenges for cell count and viability measurements for bioprocessing workflow are obtaining consistent readouts, cell counting accuracy, and the amount of time required to measure large amounts of samples.
Revvity has participated in the creation of the ISO Cell Counting Standards series and continues to provide support and practical procedures as well as protocols to practice ISO Cell Counting Standards [Bell et al. 2021, Pierce et al. 2021].
Image cytometry and automated cell counting systems modernize the cell counting and analysis workflow [Bell et al. 2021, Pierce et al. 2021]
- Improve precision, robustness, and accuracy
- Provide high-throughput analysis
Revvity image cytometry and cell counting systems can support many aspects of biologics research and development by providing rapid, user-friendly solutions for:
- Cell counting and viability
- Measuring transduction efficiency
- Cell proliferation analysis
- Functional cell cytotoxicity assays
- Apoptosis analysis
References
- Joubert S, Dodelet V, Béliard R, Durocher Y. Biomanufacturing of monoclonal antibodies. Med Sci (Paris). 2019;35(12):1153-1159. doi: 10.1051/medsci/2019219
- Dalton AC, Barton WA. Over-expression of secreted proteins from mammalian cell lines. Protein Sci. 2014;23:517-525. doi: 10.1002/pro.2439
- Fischer S, Handrick R, Otte K. The art of CHO cell engineering. A comprehensive retrospect and future perspectives. Biotechnol Adv. 2015;33:187801896. doi: 10.1016/j.biotechadv.2015.10.015
- Baeshen NA, Baeshen MN, Sheikh A, et al. Cell factories for insulin production. Microb Cell Fact. 2014;13:141. dx.doi.org/10.1186%2Fs12934-014-0141-0
- Bell et al. 2021, Characterization of a novel high-throughput, high-speed and high-precision plate-based image cytometric cell counting method, Cell and Gene Therapy Insight
- Pierce et al. 2021, Outcomes from a cell viability workshop: fit-for-purpose considerations for cell viability measurements for cellular therapeutic products, Cell and Gene Therapy Insight
For research use only. Not for use in diagnostic procedures.




























