Immunostained protein analysis
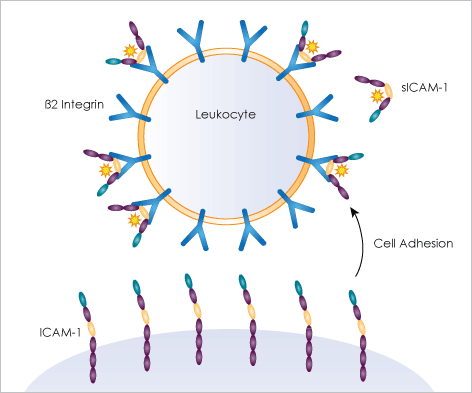
The intercellular adhesion molecule (ICAM-1) is a surface glycoprotein typically expressed in low levels on epithelial cells. It is also stimulated with IL1 and TNF-α activation to express on immune cells. Furthermore, it is a binding site on respiratory epithelial cells for the human rhinovirus (common cold virus). ICAM-1 is known for stabilizing cell-cell interaction and leukocyte endothelial transmigration.
Traditionally, live cell analysis of fluorescently-labeled ICAM-1 is performed qualitatively by inspection via fluorescent microscopy or quantitatively by flow cytometry. However, the manual microscopy method is time-consuming and non-quantitative. Flow cytometry can generate highly sensitive quantitative results but requires a highly-trained user to generatemeaningful results.
Using the Celigo image cytometer, antibody or fluorescent protein-labeled receptors can be imaged and the fluorescent intensities of the target cell populations measured to determine the level of CD54 ICAM-1 expression automatically.

Celigo imaging cytometry
Celigo operation
- Celigo images entire wells of the microplate
- The captured images are analyzed
- Results are output automatically for each analyzed sample
Experimental method
- Target cells (THP-1) are seeded at 2 x 104 cells/well in a 24-well plate (Greiner 662160)
- Cells are stimulated with different LPS concentrations
- Cells are then stained with CD54-PE and isotype control
- After staining, the plate is scanned with the Celigo system and analyzed to measure CD54 expression level on the cells
Live cell analysis of CD54 ICAM-1 fluorescent images
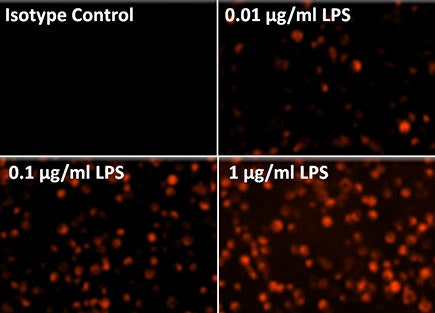
CD54 ICAM-1 staining
CD54-PE fluorescent images showed decreasing % of ICAM-1 expression on the cells as LPS concentration decreased.
The total number of cells are counted using the Celigo captured brightfield images, while the fluorescent images are counted to determine the number of cells expressing CD54 ICAM-1.
The CD54 expression level is determined by the total fluorescent intensity in each tested sample.
Quantification of CD54 expression level using Celigo image cytometer
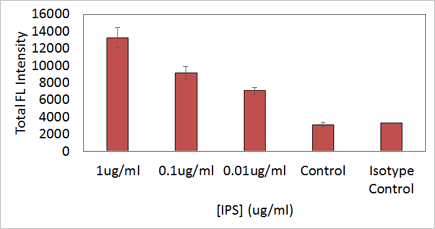
Quantification of CD54 Expression
Cells stained with ICAM-1 CD54 Ab showed decreasing fluorescent intensities as LPS concentration decreased.
Both the control with 0 ug/ml LPS and the Isotype control showed no fluorescence signals.
Conclusion
The Celigo image cytometer can be used to measure ICAM-1 CD54 expression via fluorescence detection.
The ability to capture bright field and fluorescent images in a multi-well microplate automatically allows for high-throughput cell-based assays.
In addition, analyzing adherent cells directly on the plate eliminates the need for trypsinization and removes the disruption to the cells.
Receptor Detection Assay with Transferrin
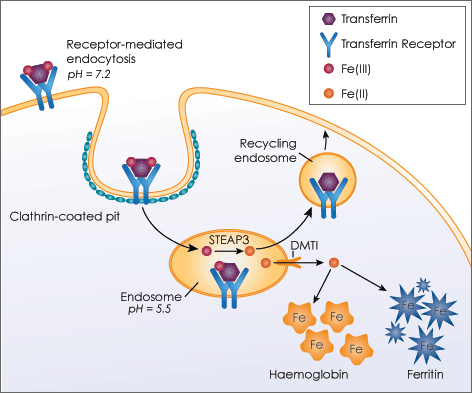
Transferrin in the blood is a glycoprotein that can transport iron to cells from the liver and intestine. Generally, the surface transferrin receptor on the cells can readily bind to the ferrotransferrin (transferrin bound with iron) at neutral pH, which then induces receptor-mediated endocytosis of the ferrotransferrin. The iron (Fe3+) ions are taken up inside the cells through the clathrin-coated vesicle into endosome, while the unbound form of apotransferrin is secreted back into the blood for reloading of iron. This pathway is an attractive delivery method for drugs, proteins and therapeutic genes into malignant cells with overexpressed transferrin receptors.
Traditionally, fluorescently-labeled transferrin receptors can be qualitatively inspected via fluorescent microscopy or quantitatively measured by flow cytometry. However, manual microscopy method is time-consuming and non-quantitative and flow cytometry can generate sensitive quantitative results, but requires a highly-trained user.
Using the Celigo image cytometer, antibody or fluorescent protein-labeled receptors can be imaged and the fluorescent intensities of the target cell populations measured to determine the level of CD71 transferrin receptor expression automatically.
Celigo imaging cytometry
Celigo operation
- Celigo images entire wells of the microplate
- The captured images are analyzed
- Results are output automatically for each analyzed sample
Experimental method
- Target cells (H376) are seeded at 2 x 104 cells/well in a 24-well plate (Greiner 662160)
- Cells are labeled with CD71-PE, isotype control, nonspecific ab, and nonspecific ab isotype
- After staining, the plate is scanned with Celigo and analyzed to measure CD71 expression level on the cells
Live cell analysis of CD71 transferrin receptor brightfield and fluorescent images
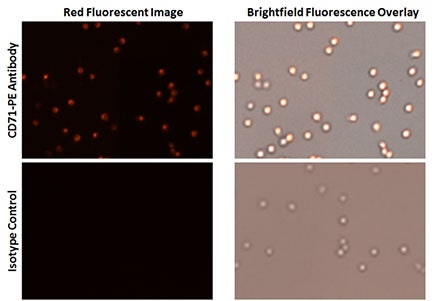
CD71-PE fluorescent images showed high % of transferrin receptor expression on the cells in comparison to the isotype control.
The total number of cells are counted using the Celigo-captured brightfield images, while the fluorescent images are counted to determine the number of cells expressing CD71 Transferrin Receptor.
The CD71 expression level is determined by the total fluorescent intensity in each tested sample
Quantification of CD71 expression level using Celigo image cytometer
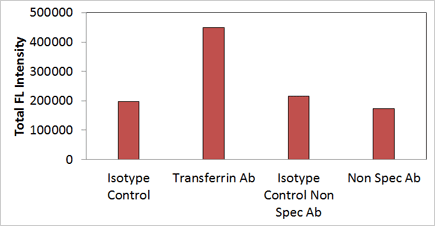
Cells stained with specific transferrin receptor Ab showed the highest total fluorescent intensity signals in comparison to the isotype control, nonspecific Ab, and isotype control for nonspecific Ab.
Conclusion
The Celigo image cytometer can be used to measure Transferrin Receptor expression via fluorescent detection.
The ability to capture brightfield and fluorescent images in a multi-well microplate automatically allows for high-throughput cell-based assays.
In addition, analyzing adherent cells directly on the plate eliminates the need for trypsinization and removes the disruption to the cells.
For research use only. Not for use in diagnostic procedures.




























