Introduction to stromal vascular fraction (SVF)
The lipoaspirate collected from adipose tissue is a valuable source of adipose tissue-derived stem cells (ADSCs) for autologous cellular therapy [1, 2, 3]. Upon the completion of liposuction, the raw lipoaspirate must be processed to obtain the stromal vascular fraction (SVF). The stromal vascular fraction from adipose tissue typically contains a heterogeneous mixture of cells, including endothelial cells, smooth muscle cells, pericytes, leukocytes, mast cells, mature adipocytes, pre-adipocytes, and adipose tissue-derived stem cells (ADSCs) [4, 5]. Counting viable cells from SVF is an important step in processing samples for cryopreservation and/or cellular therapy. Most of the constituents in the SVF are nucleated cells, which can be enumerated using the nuclear dyes acridine orange (AO), propidium iodide (PI), and Hoechst 33342.
Propidium iodide (PI) is a membrane exclusion dye that is frequently used to stain non-viable nucleated cells with compromised membranes. Acridine orange (AO) freely diffuses across the cell membrane and stains DNA in all nucleated cells. When AO and PI are combined, it is possible to determine percent viability for nucleated cells [6, 7]. Similarly, while the charged PI is excluded by live cells, Hoechst 33342 (HO) penetrates through the plasma membrane and can stain DNA in live cells [6, 7, 8]. Based on these dye characteristics, a total nucleated cell number as well as cell viability can be obtained.
Data reported here shows results from a comparative study of counting methods for SVF samples. Three detection methods were used in this experiment to analyze two separate canine SVF samples: a dual fluorescent staining method utilizing Cellometer Vision* image cytometer, a flow cytometer and manual counting using a hemocytometer.
*The Cellometer Vision instrument has been superseded by the Cellometer Spectrum.
Staining protocols for Cellometer, flow Cytometer and Hemocytometer counting
Cellometer Vision CBA* protocol
Fresh SVF samples were diluted 1:100 in PBS. The sample was mixed with staining reagent at 1:1 dilution. The Hoechst-stained sample was incubated for 45 minutes at 37°C. AO/PI was mixed with SVF sample at a 1:1 ratio and analyzed immediately.
Flow Cytometer protocol
Fresh SVF sample was diluted 1:100 in PBS. 75 µl of diluted SVF was mixed with 75 µl of HO/PI solution (2x stock) and the mixture was incubated for 45 minutes in a 37°C water bath. A total of 150 µl of CytoCount bead preparation (Dako) was added to the stained SVF samples to yield a final concentration of 1:400. The mixture was analyzed on a Synergy Cell Sorter (iCyt/Sony) by counting 10,000 beads. The ratio of beads to Hoechst-positive cells (gated on canine PBMC) was used to determine the percent of viable and dead (PI positive) cells per ml of the SVF sample.
Hemocytometer protocol
Fresh SVF sample was diluted 1:100 in PBS and subsequently 1:10 Trypan blue (TB) to yield a final concentration of 1:1000. The average of two full squares was used to calculate % viable and dead cells per ml of the SVF sample.
Data analysis
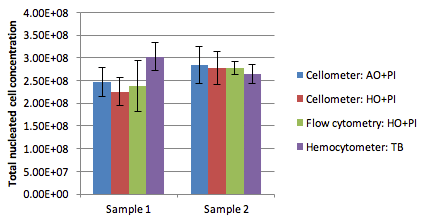
Obtained cell concentrations from each of the staining and counting methods
| Sample ID | Sample replicates | Cellometer AO+PI | Cellometer HO+PI | Flow HO+PI | Hemocytometer Trypan Blue |
|---|---|---|---|---|---|
| Sample 1 | 1 | 2.53E+08 | 2.54E+08 | 3.22E+08 | 3.00E+08 |
| Sample 1 | 2 | 2.82E+08 | 2.29E+08 | 2.01E+08 | 2.60E+08 |
| Sample 1 | 3 | 2.51E+08 | 1.94E+08 | 2.09E+08 | 3.30E+08 |
| Sample 1 | 4 | 2.04E+08 | No Data | 2.21E+08 | 3.20E+08 |
| AVE | 2.47E+08 | 2.26E+08 | 2.38E+08 | 3.03E+08 | |
| STDEV | 3.19E+07 | 3.01E+07 | 5.64E+07 | 3.10E+07 | |
| Sample 2 | 1 | 3.27E+08 | 2.50E+08 | 2.82E+08 | 2.60E+08 |
| Sample 2 | 2 | 3.05E+08 | 2.70E+08 | 2.86E+08 | 2.70E+08 |
| Sample 2 | 3 | 2.66E+08 | 3.31E+08 | 2.87E+08 | 2.90E+08 |
| Sample 2 | 4 | 2.37E+08 | 2.61E+08 | 2.57E+08 | 2.40E+08 |
| AVE | 2.84E+08 | 2.78E+08 | 2.78E+08 | 2.65E+08 | |
| STDEV | 4.04E+07 | 3.63E+07 | 1.42E+07 | 2.08E+07 | |
Consistent correlation between counting methods

Representative Cellometer images showing viability staining of SVF samples
Cellometer images of sample 1: SVF stained with AO+PI
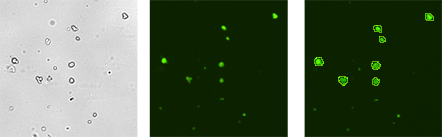
Cellometer images show brightfield (left), AO-stained (center) and AO/PI counted merged image (right). Because of high viability only live green AO positive cells are seen in the merged image.
Cellometer images of sample 2: SVF stained with HO+PI

Cellometer images show brightfield (left), Hoechst stained (center) and Hoechst + PI counted merged image (right). Because of high viability only the live nucleated cells are seen in the merged image.
Conclusion
Primary stromal vascular fractions (SVFs) are characterized by the complexity of cell types that are typically found within each sample. This complexity has some drawbacks. As seen in the above bright field images, SVF samples have a lot of cellular debris which significantly complicates the ability to acquire the correct cell count and viability.
In this study we compared the Cellometer Vision CBA* platform against manual counting using a hemocytometer as well as a flow cytometer. The results show that there is good correlation between these counting methods with the greatest similarity seen between the Cellometer platform and the flow cytometer.
The use of cell nucleus identifying dyes like AO/PI and Hoechst/PI significantly increases the confidence of proper and accurate counting and viability. Additionally, the ability to gate out small debris in both the bright field and fluorescent channels assures that the correct cell count is obtained.
With the ability to perform rapid staining (AO/PI staining is immediate) and analysis (<30 seconds per sample for imaging and analysis) while using a small sample volume (20 µL) the Cellometer Vision CBA* supports the determination of cell concentration and viability in stromal vascular fractions.
*The Cellometer Vision CBA instrument has been superseded by the Cellometer Spectrum.
References
- Patricia A. Zuk, et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 14, 4279-4295 (2002)
- Patricia A. Zuk, The adipose-derived stem cell: looking back and looking ahead. Molecular Biology of the Cell. 21, 1783-1787 (2010)
- Benjamin Levi, Michael T. Longaker, Adipose derived stromal cells for skeletal regenerative medicine. Stem Cells. 29(4), 576-582 (2011)
- Wouter J. F. Jurgens, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell and Tissue Res. 332, 415-426 (2008)
- Andreas Schäffler, Christa Büchler, Consise review: Adipose tissue-derived stromal cells – basic and clinical implications for novel cell-based therapies. Stem Cells. 25, 818-827 (2007)
- Zbigniew Darzynkiewicz, et al. Essential Cytometry Methods. (Academic Press, first edition 2009)
- Howard M. Shapiro. Practical Flow Cytometry. (Wiley, New Jersey, ed. 4, 2003), chap. 3, 7.
- K. Cai, et al. Single UV excitation of Hoechst 33342 and propidium iodide for viability assessment of rhesus monkey spermatozoa using flow cytometry. Archives of Andrology. 51(5), 371-383 (2005)
For research use only. Not for use in diagnostic procedures.




























