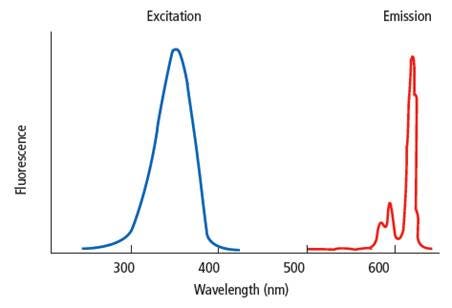
Overview
Fluorescence is the emission of light by a substance that results from energy acquired when the substance is excited using light at a different wavelength than the emission signal. In fluorescence assays, the chemical or dye that fluoresces is referred to as a "fluorophore". Each fluorophore has its own unique spectral properties; it will require excitation at a particular wavelength and will release light at a particular emission wavelength. The excitation and emission wavelengths are not discrete wavelengths, but rather a range of wavelengths characteristic to a given fluorophore. In a typical fluorescent assay, the fluorophore is excited by light at a given wavelength, and the signal at an emission wavelength is measured using a plate reader.

Examples of fluorescence assays include fluorescence intensity (FI) assays, fluorescence polarization (FP) assays, FRET assays (Förster resonance energy transfer assays), fluorescent calcium flux assays, TRF assays (time-resolved fluorescence assays, including DELFIA), and TR-FRET assays (time-resolved Förster resonance energy transfer assays, including HTRF™ and LANCE™ Ultra™).
Black vs. white plates
Autofluorescence
Autofluorescence is fluorescence resulting from substances other than the fluorophore-of-interest, and can negatively affect an assay by increasing background signal. Many components of assay buffers and biological samples can autofluoresce. Autofluorescence is triggered by the same excitation light used to excite the fluorophore in the fluorescence assay. The severity of background autofluorescence can vary based on the excitation wavelength being used in a particular assay. For example, higher excitation wavelengths (above 650 nm) usually cause less autofluorescence than wavelengths in the UV/Vis range.
Because white plates reflect light and black plates tend to quench light, background fluorescence will be higher in white plates as opposed to black plates. For this reason, black plates are typically recommended for fluorescence assay that use short half-life fluorophores. Time-resolved fluorescence assay, which use longer half-life fluorophores, can use either white or black plates (read below for more information).
General fluorescence assays
General fluorescence assays include fluorescence intensity (FI), fluorescence polarization, and FRET assays. These assays use traditional fluorophores such as fluorescein, cyanine 3, cyanine 5, green fluorescent protein (GFP), rhodamine, Texas Red, coumarin, and other fluorophores. These fluorophores have relativelyshort half-lives (< µsec, for microplate-based assays). We recommend running assays utilizing short half-life fluorophores in black plates to reduce background autofluorescence.
Time-resolved fluorescence assays
Time-resolved fluorescence assays, including both standard TRF and TR-FRET assays, involve fluorophores that have longer half-lives (µsec – msec, for microplate-based assays). Examples of such fluorophores include Europium chelates and cryptates, Samarium chelates, and Terbium chelates and cryptates. Because of the longer half-life of the fluorescent signal, you can set up your instrument to incorporate a "lag time" or "delay time" between the time the fluorophore is excited, and the time you begin reading the emission signal (time-resolved mode). This allows background autofluorescence to fade before you begin collecting emission signal from assays involving a long half-life fluorophore. Because of this, time-resolved fluorescence assays can be run in either black or white plates. The use of white plates will result in higher raw signals, because the light is reflected maximally by the white color of the plate. The use of black plates will result in lower raw signals, because the black color of the plate can quench the light. However, black plates may help in situations where cross-talk is an issue, resulting in better sensitivity. In general, we recommend white plates for higher-density time-resolved fluorescence assays (for example, assays run in 384-well and 1536-well plates) and other assays where a lower signal might be expected.
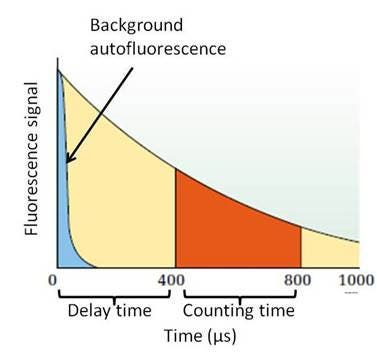
Figure 2. Measurement of a long half-life fluorophore. The fluorescent signal for this particular fluorescent reagent lasts longer than 1000 μs, allowing for a time-resolved read. The fluorophore is excited, which also produces background autofluorescence. This autofluorescence fades quickly in relation to the signal from the long half-life fluorophore, allowing you to use a delay time before collecting emission signal to avoid collecting signal from autofluorescence.
Plate density considerations
Fluorescent signal is higher in white plates because the light is reflected maximally by the white color of the plate. The use of black plates can quench the light, leading to a reduced signal. For assays using long half-life fluorophores (for example, HTRF™, LANCE™Ultra™, and DELFIA™ assays), you could potentially use either white or black plates. However, as the plate density increases (384-well and 1536-well plates are "higher density" plates; 96-well plates are "lower density" plates), the use of white plates becomes more advantageous. This is due to the volumes used in these assay formats; assays run in 1536-well format are typically performed in 4-10 μL assay volumes. There is comparatively less fluorophore used in these assays, and maximizing signal may become more important as the amount of fluorophore in the well decreases.
Yellow DELFIA plates
DELFIA yellow plates are semi-translucent yellow plates developed to have very low background autofluorescence in order to give optimal sensitivity in DELFIA TRF assays. In addition to providing increased sensitivity in many DELFIA assays, the DELFIA yellow plates are strongly recommended when running multiplexed DELFIA TRF assays (assays involving more than one DELFIA lanthanide - for example, Europium and Terbium chelates, or Europium and Samarium chelates, etc.)
DELFIA yellow plate
Microplates for cell-based fluorescence assays
The optimal microplate for a cell-based assay will be dependent on both the specific cell line being used in the assay and the assay protocol itself. Questions that need to be answered in choosing the plate include:
- Do I need a sterile, tissue culture-treated plate?
- Does the plate need to be coated?
- Should the plate have a clear or opaque bottom?
Plate considerations
Whether or not it is necessary to use a sterile, tissue culture-treated plate depends on the length of time the cells are going to be in the assay plate. In some assays the cells are added to the microplate and the assay is completed within a few minutes to a few hours. In other cases, cells are grown in plates at least overnight prior to performing the assay or are treated with compounds for extended lengths of time. As a general recommendation, if the assay is going to be performed within a single working day a sterile, tissue culture-treated plate is not necessary. If the cells are going to be in the plate overnight or longer a sterile, tissue culture-treated plate should be used, and aseptic techniques should be followed.
The need for tissue culture-treated or coated plates depends on the specific cell lines used, and how the cells are going to be treated in the course of the assay. Cells can be broadly divided into three classes of cells:
- Strongly adherent cells
- Poorly adherent cells
- Suspension or non-adherent cells
Our sterile microplates are all tissue culture-treated to promote cell attachment and growth. The tissue culture treatment process involves exposing a polystyrene microplate to a plasma gas in order to modify the hydrophobic plastic surface to make it more hydrophilic. The resulting surface carries a net negative charge due to the presence of oxygen-containing functional groups such as hydroxyl and carboxyl. Strongly adherent cells will usually attach satisfactorily to tissue culture-treated plates. Cell lines that attach less strongly may require a plate with a coating such as poly-D-lysine or collagen which promotes attachment better than just tissue culture treatment. Cell-based assays using suspension cells are generally performed in standard tissue culture-treated plates. Coated plates are not typically used with suspension cells.
In addition to the specific cell line being used in the assay, the assay protocol itself is important in deciding the type of plate to use. For example, assays using adherent cells may include culture medium changes or wash steps in the protocol. In such cases it may be advisable to use a coated plate for the assay in order to prevent the cells from becoming detached from the plate during the assay.
Clear bottom vs. opaque plates
Microplates with clear bottoms can be useful for cell-based assays as they allow the microscopic visualization of the cells to monitor confluency, morphology and other parameters that may affect the cellular response in the assay. In addition, assays that are configured for bottom reading require clear bottom plates.
Clear bottom plates can be converted to functionally opaque plates by application of a BackSeal™ Adhesive Bottom Seal. BackSeal plate seals are available in either white or black (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seal should match the color of the sides of the plate wells.
Selection table for cell-based fluorescence assays
| Plate | Description |
|---|---|
| CulturPlate |
|
| ProxiPlate |
|
| ViewPlate |
|
| CellCarrier |
|
Clear-bottom, TC-treated IsoPlate microplates could also be used for cell-based fluorescent assays. Clear-bottom IsoPlate microplates are similar to ViewPlate microplates in that the bottom of the plate is clear, while the sides of each well are either black or white. This makes the IsoPlate microplate suitable for bottom-reading instruments. However, there are a few differences between IsoPlate and ViewPlate microplates. IsoPlate microplates are manufactured by first molding 96 clear wells at a time, then molding a black or white frame around the clear wells. This makes the white- or black-colored well extend to the same depth as the clear well base and can help reduce cross-talk in bottom-reading assays. IsoPlates were originally developed for coincidence counting in a MicroBeta® radiometric detection instrument (reading from and bottom coincidentally). While they have clear bottoms, IsoPlates are not ideal for confocal imaging (microscopic observations) because the optical clarity of the bottom is not as good as clarity is with CellCarrier™ Ultra or ViewPlate microplates. Additionally, IsoPlates are only available in a 96-well format.
Tips and FAQs
- BackSeal plate seal can be used to convert a clear-bottom plate into an opaque (solid-colored) plate for reads. BackSeal plate seal is offered in both white (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seal should match the color of the sides of the plate wells.
Q. What kind of lid can I use for my plates?
A. TC-treated CulturPlates, ViewPlates, and ProxiPlates, as well as all TC-treated microplates from Revvity, are supplied with a lid. The exception to this is when the TC-culture treated plate is purchased in a case of 200. In this instance, lids are not supplied with the microplates and much be purchased separately. For 96-well plates, the catalog number for the lid is 6005619, and for 384-well and 1536-well plates the catalog number for the lid is 6007619. Lids for 96-well plates have condensation rings that align with the underlying wells. These lids will leave a small space between the lid and the well. This is necessary so cells can ‘breathe’ when growing. Lids should be removed prior to reading.
Q. Can I use a seal on my plates, or will that kill my cells?
A. If cell viability is no longer an issue, Seal-A™ adhesive plate seal (catalog number 6050185) can be used to prevent evaporation during incubation steps. The plastic of the Seal-A adhesive seal has some spectral properties that may interfere with assays performed at certain excitation or emission wavelengths. It may be necessary to remove the seal from the plate prior to reading. If cell viability is an issue at the time a seal is needed, we recommend using sterile plate lids (if sterile practices need to be maintained) or breathable plate seal (if antibiotics/antifungals can be added to the culture media to prevent contamination). Breathable plate seals are available from various suppliers, including Nunc® and Corning®.
Microplates for biochemical fluorescence assays
Microplates for standard, in vitro fluorescent assays that do not require anchoring of cells or other reagents to the surface of the plate.
| Plate | Description |
|---|---|
| OptiPlate™ |
|
| ProxiPlate™ |
|
| 1/2 AreaPlate™ |
|
| ViewPlate™ (non-TC treated) |
|
| IsoPlate™ |
|
| VisiPlate™ (non-TC treated) |
|
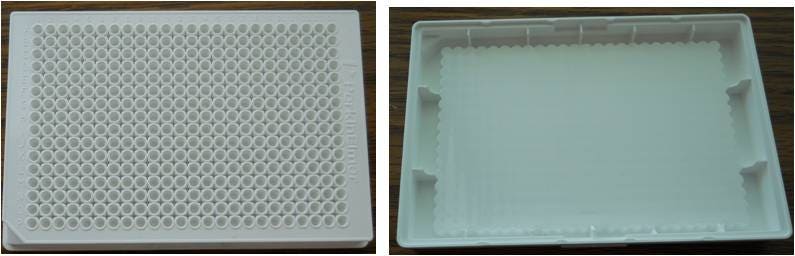
Figure 3: view of a 384-well white ProxiPlate microplate (left) and view from underneath the same plate (right). The bottom of the wells is pushed towards the surface of the plate to help increase signal while allowing use of low assay volumes.

Figure 4: 96-well OptiPlate microplate (left) vs. 96-well ½ AreaPlate microplate (right)

Figure 5: Photo from the bottom of a 96-well ViewPlate microplate (left) and a 96-well clear-bottom IsoPlate microplate (right). The ViewPlate microplate has a one-piece rectangular clear plastic base on the underside of the plate. The IsoPlate microplate has individual circular clear plastic well bottoms that are planar with the bottom of the white- or black-framed wells.
Clear-bottom IsoPlate microplates are similar to ViewPlate microplates in that the bottom of the plate is clear, while the sides of each well are either black or white. This makes the IsoPlate microplate suitable for bottom-reading instruments. However, there are a few differences between IsoPlate and ViewPlate microplates. IsoPlates are manufactured by first molding 96 clear wells at a time, then molding a black or white frame around the clear wells. This makes the white- or black-colored well extend to the same depth as the clear well base and can help reduce cross-talk in bottom-reading assays. IsoPlates were originally developed for coincidence counting in a MicroBeta™ radiometric detection instrument (reading from and bottom coincidentally). While they have clear bottoms, IsoPlates are not ideal for confocal imaging (microscopic observations) because the optical clarity of the bottom is not as good as clarity is with CellCarrier™ Ultra or ViewPlate microplates. Additionally, Isoplates are only available in a 96-well formation while ViewPlates are available in 96-, 384-, and 1536-well formats.
Tips and FAQS
- BackSeal plate seal can be used to convert a clear-bottom plate into an opaque (solid-colored) plate for reads. BackSeal plate seal is offered (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seal should match the color of the sides of the plate wells.
Q. What kind of plate seal can I use for my plates?
A. Seal-A adhesive plate seal (catalog number 6050185) can be used to prevent evaporation during incubation steps. The plastic of the Seal-A adhesive seal has some spectral properties that may interfere with assays performed at certain excitation or emission wavelengths. It may be necessary to remove the seal from the plate prior to reading.
Q. What kind of lids can I use for my plates?
A. Clear, sterile lids to fit our microplates can be ordered separately. For 96-well plates, the catalog number for the lid is 6005619, and for 384-well and 1536-well plates the catalog number for the lid is 6007619. Lids for 96-well plates have condensation rings that align with the underlying wells. These lids will leave a small space between the lid and the well. This is necessary so cells can ‘breathe’ when growing. Lids should be removed prior to reading.
Microplates for coated-plate assays
Sometimes referred to as "solid phase assays", coated-plate assays require the anchoring of one of the assay components (protein, antibody, sample, etc.) to the surface of the microplate. Coated-plate assays use wash steps to separate bound (associating) and unbound (non-associating) reagents from the well of the plate.
| Plate | Description |
|---|---|
| OptiPlate™ HB |
|
| IsoPlate™ HB |
|
| DELFIA™ uncoated plates |
|
| DELFIA™ pre-coated plates |
|
Tips and FAQS
- BackSeal plate seal can be used to convert a clear-bottom plate into an opaque (solid-colored) plate for reads. BackSeal plate seal is offered (catalog number 6005199 for white, catalog number 6005189 for black). The color of the BackSeal plate seals should match the color of the sides of the plate well walls.
Q. What kind of plate seal can I use for my plates?
A. Seal-A adhesive plate seal (catalog number 6050185) can be used to prevent evaporation during incubation steps. The plastic of the Seal-A adhesive seal has some spectral properties that may interfere with assays performed at certain excitation or emission wavelengths. It may be necessary to remove the seal from the plate prior to reading.
Q. What kind of lids can I use for my plates?
A. Clear, sterile lids to fit our microplates can be ordered separately. For 96-well plates, the catalog number for the lid is 6005619, and for 384-well and 1536-well plates the catalog number for the lid is 6007619. Lids for 96-well plates have condensation rings that align with the underlying wells. These lids will leave a small space between the lid and the well. This is necessary so cells can ‘breathe’ when growing. Lids should be removed prior to reading.
Q. Do you have any suggested plate coating protocols that I can use to bind my antibody/sample to the plate?
A. High-bind plates can be coated using any standard plate-coating method. Plates can be coated passively using the basic outline below:
- Antibody, protein, or sample (concentration of ~10 µg/mL or higher) is incubated in the plate overnight at an appropriate temperature (room temperature or 4 °C). Select a temperature that will help maintain stability of the antibody, protein, or sample being coated.
- Plate is washed three times with buffer (for example, 1X PBS).
- Plate is "blocked" overnight to cover the well surface area that remains (typically using BSA, sugars such as trehalose or casein, serum, etc.)
- Final washes are performed with buffer before using the plate in an assay.
For more information on plate coating, blocking, and storage, we recommend this reference:
Brown, M. C. (2011) Microtiter Plate Elisa, in Immunoassays in Agricultural Biotechnology (ed G. Shan), John Wiley & Sons, Inc., Hoboken, NJ, USA. doi: 10.1002/9780470909935.ch4
A look at plate performance using Revvity fluorescence assays
LANCE™ Ultra™ TR-FRET assays
LANCE Ultra assays are TR-FRET assays using a Europium chelate as the donor fluorophore. Either white or black plates indicated for fluorescence applications can be used, though we typically recommend white plates for LANCE Ultra assays. LANCE Ultra assays utilize time-resolved fluorescence resonance energy transfer technology (TR-FRET) to generate a fluorescent signal at 665 nm. In contrast to prompt fluorescence assays, TR-FRET assays delay the signal acquisition for 50 µseconds in order to let any autofluorescence signal from the microplate or sample decay to background. Revvity recommends white OptiPlate™ microplates for LANCE Ultra assays, both LANCE Ultra cAMP and LANCE Ultra kinase. Black microplates are generally used for prompt fluorescence assays to avoid the high autofluorescence generated by white plates. Black plates may also be preferred over white plates in situations where the assay generates a relatively high signal level that may cause well-to-well cross-talk in white plates. The disadvantage of using black plates is that a significant amount of the assay signal is absorbed by a black plate compared to a white plate. We have demonstrated the superior performance of white plates in LANCE Ultra assays by comparing:
- The performance of the LANCE Ultra cAMP assay in white and black 384-well OptiPlate microplates.
- In addition, we used a LANCE positive control to see if there is any issue with well-to-well cross-talk signal in both 1536- and 384-well white OptiPlate microplates.
Materials and Methods
| Product Name | Revvity Product Number |
|---|---|
| OptiPlate-384 microplate, white | 6007290 |
| OptiPlate-384 F microplate, black | 6007270 |
| ProxiPlate-384 Plus microplate, white, shallow well | 6004290 |
| OptiPlate-1536 microplates, white opaque | 6004290 |
| LANCE Ultra cAMP kit | TRF0262 |
Standard cAMP curves were generated following the instructions in the LANCE Ultra cAMP kit. Samples were measured in triplicate in 384-well white and black OptiPlate microplates. The total assay volume was 20 µL per well. The data was analyzed using GraphPad Prism® software.
The LANCE positive control was used as directed to generate the signal for the cross-talk experiments. Wells not containing the LANCE control contained PBS. Cross-talk was measured in both adjacent and diagonal wells according to the platemap shown below.
| Blank | Diagonal | Adjacent | Diagonal | Blank |
| Blank | Adjacent | LANCE Control | Adjacent | Blank |
| Blank | Diagonal | Adjacent | Diagonal | Blank |
The volume of LANCE control added was:
- 1536-well Optiplate microplate - 5 µL
- 384-well OptiPlate microplate - 20 µL
- 344-well ProxiPlate microplate - 10 µL
The volumes used are representative of typical assay volumes for these plate types.
Plates were read on the EnVision™ Multimode Plate Reader using the following settings:
| Light Source | Flash lamp |
|---|---|
| Mirror | LANCE/DELFIA Dual |
| Excitation Filter | UV (TRF) 320 |
| Emission Filter | APC 665 |
| Cycle | 200 |
| Delay | 50 |
| Number of flashes | 100 |
Results
The standard curves measured in the white and black 384-well OptiPlate microplates are plotted in Figure 1. Curve-fitting data is summarized in the table below the graph.
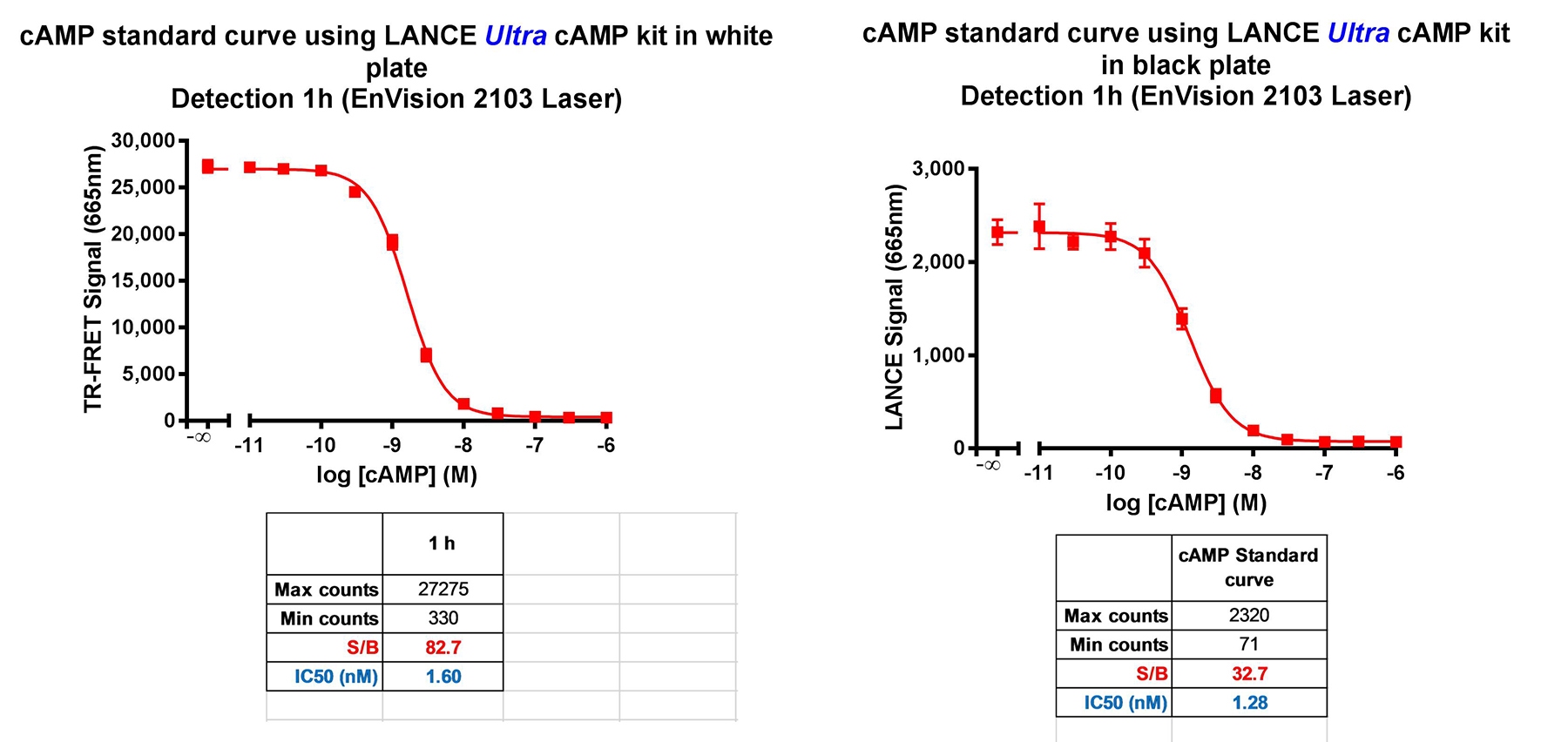
Figure 5. LANCE Ultra cAMP standard curves generated in white and black 384-well OptiPlate microplates.
The two standard curves gave similar EC50 and HillSlope values. The signal measured using a white plate was approximately 10-fold greater than that from the black plate, and the S/B ratio was also better using a white plate.
The well-to-well crosstalk measured in the various plate formats is shown in the table below.
| OptiPlate | ProxiPlate | OptiPlate | |
|---|---|---|---|
| Plate format | 384-well | 384-well | 1536-well |
| Positive Control | 822,176 | 539,231 | 207,325 |
| Blank | 496 | 477 | 790 |
| Adjacent cross-talk | 461 | 502 | 837 |
| Diagonal cross-talk | 461 | 502 | 837 |
| Adjacent % cross-talk | 0.004 | 0.005 | 0.02 |
| Diagonal % cross-talk | 0.003 | 0.002 | 0.02 |
Conclusions
White OptiPlate microplates are clearly superior to black OptiPlate microplates for performing LANCE Ultra assays. White plates give a much higher assay signal, and also a better S/B ratio than black plates. Autofluorescence from a white microplate is very low due to the time-resolved nature of the detection technology. Cross-talk in LANCE Ultra assays using white plates is not an issue, even in 1536-well format.
Recommended microplates for LANCE Ultra assays
White plates are recommended in all cases for performing LANCE Ultra assays. Depending on the desired well density and assay volume the following plates are suitable for LANCE Ultra assays:
- 384-well white Optiplate, CulturPlate and ProxiPlate microplates
- 1536-well white OptiPlate and CulturPlate microplates
- 96-well white OptiPlate, CulturPlate, ProxiPlate and ½-AreaPlate microplates
DELFIA™ TRF assays
DELFIA assays are TRF assays using a Europium chelate, Terbium chelate, and/or Samarium chelate as the assay fluorophore. White, black, clear, or "DELFIA yellow" microplates are recommended for DELFIA assays. DELFIA yellow plates are semi-translucent yellow plates developed to give optimal sensitivity in DELFIA assays. DELFIA yellow plates are strongly recommended for multiplexed DELFIA assays.
The signal to background ratio obtained using a 96-well yellow DELFIA plate (catalog number AAAND-001) was compared to that of a clear 96-well SpectraPlate microplate. A standard sample of 1 nM europium in DELFIA enhancement solution (in triplicate) was compared to a background sample of wells filled with PBS. The results shown in the table below demonstrate that the low background signal generated by the yellow DELFIA plates leads to a much higher S/B ratio.
| Yellow DELFIA plate | Clear SpectraPlate microplate | |
|---|---|---|
| Average Signal | 266,011 | 242,130 |
| Average Background | 135 | 871 |
| S/B Ratio | 1,975 | 278 |
Plate Seals
Revvity offers a variety of plate seals. Seal™ is a range of plate seals that are applied to the surface of the plate and are used to prevent evaporation or radioactive contamination during assay incubation steps and/or plate reading measurements. Seal-A can be left on the plate during luminescence, AlphaScreen™, AlphaLISA™, AlphaLISA™ SureFire® Ultra™, and radiometric measurements. Seal plate seals have spectral properties that may interfere with other types of assay measurements (absorbance assays, colorimetric assays, fluorescence assays). For these types of assays, you should compare the plate measurement with and without a Seal plate seal to test for interference. BackSeal plate seals are plate seals that are applied to the bottom of the plate. BackSeal plate seals can be used to seal the bottom of a filter plate prior to the addition of scintillation cocktail, preventing leakage. BackSeal plate seals can also be used to change a clear-bottom plate into a white- or black-bottom plate in order to reduce cross-talk during -reading measurements.
Plate Seal Products
| Product | Type of Seal | Plate Format | Number of Seals | Catalog Number |
|---|---|---|---|---|
| Seal-A | Clear adhesive seal | All | 100 | 6050185 |
| Clear adhesive seal, for 24-well plates | 24-well | 100 | 6005189 | |
| Black adhesive seal | All | 100 | 6050173 | |
| Seal-B | Adhesive seal for PCR plates | All | 100 | 6050174 |
| BackSeal | White adhesive seal | 96-, 384- well | 55 | 6005199 |
| Black adhesive seal | 96-, 384- well | 55 | 6005189 |
Custom plate services at Revvity
Revvity offers custom microplates services, including:
- Bulk ordering
- Fast and flexible plate barcoding
- Biological plate coating including – poly-D-lysin, collagen, streptavidin, and antibody coating
- Custom treatments including – tissue-culture, high protein binding and low protein binding
- Customer sterilization
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.




























