Introduction
The hemocytometer has been an essential tool for hematologists, medical practitioners, biologists and now brewers and ethanol production researchers. Yeasts are an economically important organism used for ethanol production, in the beverage and alternative fuels industries as well as a leavening agent in the baking industry. Concentration and viability determinations are routinely performed for quality control purposes in yeast production, fermentation processes, and fungicides research to monitor proliferation of pathogenic yeasts.
Manual yeast cell counting with a Hemocytometer
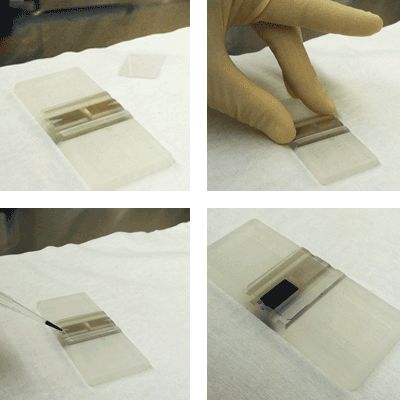
Step 1. Prepare the Hemocytometer

Clean the hemocytometer and glass cover slip with 70% EtOH.
Step 2. Prep sample & load

Place the glass cover slip over the counting chambers.

Pipette 10 microliters of cell sample into the hemocytometer.
Step 3. Manually count cells in sample
Place the hemocytometer under a microscope with a typical magnification of 100.
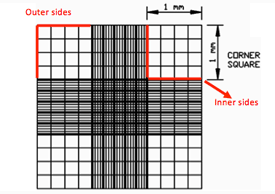
Focus both onto the grid pattern and the cell particles and count the total number of cells found in 4 large corner squares.
If cells are touching the 4 perimeter sides of a corner square, only count cells on 2 sides, either the 2 outer sides or 2 inner sides.
Step 4. Cell calculations & disposal of Hemocytometer
Multiply the dilution factor by the total number of cells, divide by the # of corner squares counted, and multiply by 104 to obtain cell concentration (cells/ml).
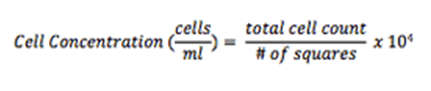
Clean hemocytometer and glass cover slip with 70% EtOH.
Manual yeast cell viability measurement with Hemocytometer
Using methylene blue to measure yeast cell viability
In general, methylene blue is used to measure yeast viability/vitality. Methylene blue is a metachromatic stain that has a molecular mass of 319.85 g/mol. Metabolically active viable/vital yeast cells with dehydrogenase activity can convert the methylene blue to a colorless substance, while the dead cells retain the blue color of the stain. Therefore, live and dead yeast cells can be manually counted using the hemocytometer to determine yeast cell concentration and viability.
Methylene blue protocol
Methylene blue preparation
Step 1. Dissolve methylene blue in sodium citrate solution (2% w/v) to a final concentration 0.01% (w/v)
Step 2. Filter the methylene blue with 0.2 micron filter
Hemocytometer preparation
Step 3. Clean the hemocytometer and glass cover slip with 70% EtOH
Step 4. Place the glass cover slip over the counting chambers
Yeast cell counting procedure
Step 5. Vortex the target yeast cell suspension and mix 1:1 with 0.01% methylene blue
Step 6. Pipette 10 microliters of cell sample into the hemocytometer
Step 7. Wait 60 seconds for the cells to settle
Manually count cells in sample
Step 8. Place the hemocytometer under a microscope with a typical magnification of 100
Step 9. Focus both onto the grid pattern and the cell particles and count the total number of cells found in 4 large corner squares. If cells are touching the 4 perimeter sides of a corner square, only count cells on 2 sides, either the 2 outer sides or 2 inner sides
Step 10. Count the live yeast cells (without methylene blue) and dead yeast cells (with methylene blue)
Cell calculations & disposal of Hemocytometer
Step 11. Multiply the dilution factor by the total number of cells, divide by the # of corner squares counted, and multiply by 104 to obtain cell concentration (cells/ml)
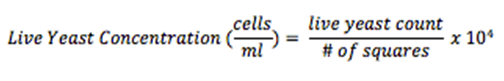
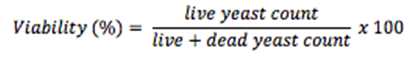
Step 12. Clean hemocytometer and glass cover slip with 70% EtOH
Crystal violet protocol for total nuclei counting
Crystal violet protocol
Crystal violet preparation
Step 1. Prepare 0.1 M citric acid by dissolving 1.9212 g in 100 mL distilled water
Step 2. Prepare 0.1 M citric acid containing 0.01% (w/v) crystal violet by dissolving 0.005 g crystal violet (also known as basic violet 3 or gentian violet; C.I. 42555) in 50 mL of the 0.1 M citric acid prepared in Step 1
Hemocytometer preparation
Step 3. Clean the hemocytometer and glass cover slip with 70% EtOH
Step 4. Place the glass cover slip over the counting chambers

Yeast Cell Counting Procedure
Step 5. Centrifuge target cell suspension at 500 ± 50 g for 5 to 10 minutes.
Step 6. Decant supernatant. Add 1.0 mL 0.1M citric acid solution to the cell pellet. Mix well and incubate at 35° C for 1 to 2 hours
Step 7. Separate nuclei by violent shaking followed by centrifugation at 1000 ± 100 g for 20 to 25 minutes
Step 8. Discard supernatant. Re-suspend the cell pellet in 0.5 to 1.0 mL citric acid-crystal violet solution
Step 9. Pipette 10 microliters of cell sample into the hemocytometer
Step 10. Wait 60 seconds for the cells to settle
Manually Count Nuclei in Sample
Step 11. Place the hemocytometer under a microscope with a typical magnification of 100
Step 12. Focus both onto the grid pattern and the cell particles, and count the total number of nuclei found in 4 large corner squares
Step 13. If nuclei are touching the 4 perimeter sides of a corner square, only count cells on 2 sides, either the 2 outer sides or 2 inner sides
Step 14. Count the live nuclei (with crystal violet)
Nuclei Calculations & Cleaning of Hemocytometer
Step 15. Multiply the dilution factor by the total number of nuclei, divide by the # of corner squares counted, and multiply by 104 to obtain cell concentration (cells/ml)
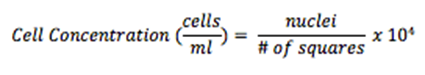
Step 16. Clean hemocytometer and glass cover slip with 70% EtOH
For research use only. Not for use in diagnostic procedures.




























